

Background: Olokizumab (OKZ), an IL-6 ligand inhibitor, demonstrated significant improvements in signs and symptoms of RA vs placebo (PL) and non-inferiority to adalimumab (ADA) in doses of 64 mg every two weeks (q2w) or every 4 weeks (q4w). 1-3 Patients (pts) who completed core phase III studies, were enrolled in an open-label extension study (OLE) (ClinicalTrials.gov number, NCT03120949).
Objectives: To report long-term safety and efficacy results of OKZ in combination with MTX.
Methods: During the OLE study, pts continued OKZ 64 mg q2w or q4w, and those who were on PBO or ADA were randomized to OKZ 64 mg q2w or q4w.
Adverse events (AE) were assessed through 82 weeks of the OLE treatment and an additional 20 weeks of safety follow-up. All AEs were analyzed using descriptive statistics.
Efficacy assessments included proportions of pts who achieved ACR20/50/70 responses, SDAI ≤3.3, improvement in HAQ-DI ≥0.22, mean change in CDAI, and DAS-28-CRP using descriptive statistics.
Results: 2105 eligible pts were enrolled, 1709 (81.2%) pts completed the OLE treatment period and 394 (18.7%) pts discontinued treatment, mainly due to adverse events 180 (8.6%), due to subject decision 158 (7.5%) and due to lack of efficacy 9 (0.4%).
Treatment emergent AE occurred in 1546 (73.5%) pts, the most common AE 809 pts (38.5%) were infections (
Serious AE were reported by 249 (11.8%) pts, serious infections - by 87 (4.1%) pts.
Deaths occurred in 1.2% of pts, equally distributed between the two groups with the most frequent reason being infections.
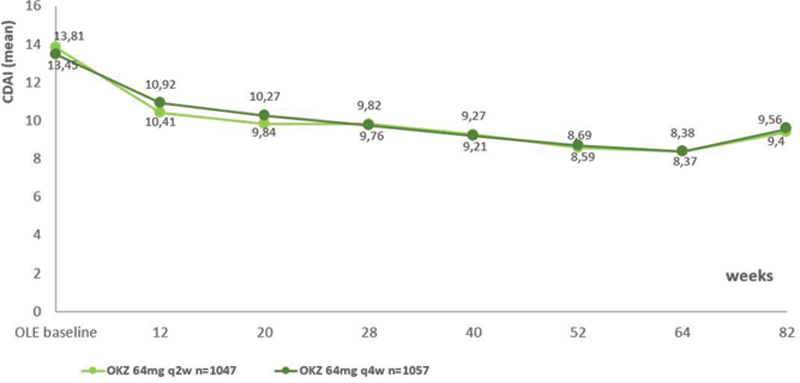
Efficacy of OKZ was maintained throughout the OLE (
Mean CDAI over time (modified Intent-to-treat population). Abbreviations : CDAI, clinical disease activity index.
There were no apparent differences in safety and efficacy outcomes between OKZ arms, except incidence of major adverse cardiovascular events appeared slightly higher in q4w arm (
Anti-drug antibodies occurred infrequently and neutralizing antibodies were rare.
Conclusion: OKZ was safe and well-tolerated that is consistent with the results of previously reported studies of OKZ and IL-6 inhibitors. Efficacy of OKZ was maintained through Wk 82 and discontinuation rates were low.
REFERENCES:
[1]Feist E, et al. Arthritis Rheumatol. 2021; 73 (suppl 10): 3508-10;
[2]Feist E, et al. Arthritis Rheumatol. 2021; 73 (suppl 10): 3510-13;
| All cases, n (%) | OKZ 64 mg q2w (OLE), N=1061 | OKZ 64 mg q4w (OLE), N=1043 |
|---|---|---|
| Any AE | 793 (74.7%) | 753 (72.2%) |
| Any serious AE | 120 (11.3%) | 129 (12.4%) |
| Any AE leading to discontinuation of study drug | 90 (8.5%) | 87 (8.3%) |
| Any death | 13 (1.2%) | 13 (1.2%) |
| Any AE of special interest | 611 (57.6%) | 598 (57.3%) |
| Infections | 398 (37.5%) | 389 (37.3%) |
| Opportunistic infections | 22 (2.1%) | 19 (1.8%) |
| Malignancies | 6 (0.6%) | 9 (0.9%) |
| Basal cell carcinomas | 1 (<0.1%) | 2 (0.2%) |
| Hyperlipidemia | 138 (13.0%) | 120 (11.5%) |
| Systemic injection and hypersensitivity reactions | 100 (9.4%) | 99 (9.5%) |
| Anaphylactic reactions | 0 | 0 |
| Gastrointestinal perforation | 2 (0.2%) | 1 (<0.1%) |
| Neutro-, thrombocyte-, leukocyte-, pan-cytopenias | 168 (15.8%) | 146 (14.0%) |
| Potential hepatotoxicity (ALT >3 ULN) | 63 (5.9%) | 68 (6.5%) |
| Injection site reactions | 34 (3.2%) | 34 (3.3%) |
| Demyelination in peripheral or central nervous system | 1 (<0.1%) | 0 |
| Autoimmune disorders | 41 (3.9%) | 48 (4.6%) |
| Serious infection | 47 (4.4%) | 40 (3.8%) |
| Any potential adjudicated major adverse cardiac events | 7 (0.7%) | 15 (1.4) |
AE, adverse event; ALT, alanine aminotransferase; n, number of subjects; %, percentage of subjects calculated relative to the total number (N) of subjects in the population; ULN, upper limit normal
[3]Nasonov E, et al. Ann Rheum Dis. 2021; 0:1–11
Acknowledgements: R-Pharm funded this study; contributed to its design; participated in data collection, analysis, and interpretation of the data; and in the writing, review, and approval of the abstract. No honoraria or payments were made for authorship. Medical writing support was provided by Sofia Kuzkina, MD, (Moscow, RF) R-Pharm employee.
Disclosure of Interests: Eugen Feist Consultant of: Abbvie, BMS, Eli Lilly, Gilead Sciences, Galapagos, Medac, Novartis, Roche, Sanofi, Sobi, R-Pharm, Grant/research support from: BMS, Eli Lilly, Novartis, Roche, Evgeny Nasonov Consultant of: AbbVie, Eli Lilly, Janssen, Novartis, Pfizer, Michael Luggen Grant/research support from: Abbvie, Biogen, GSK, Lilly, R-Pharm, Sun Pharma, and UCB, Saeed Fatenejad Shareholder of: Pfizer, INC, Consultant of: ICON and PPD contract research organizations, R-Pharm, Sergey Grishin Employee of: R-Pharm, Mikhail Samsonov Employee of: R-Pharm, Roy M. Fleischmann Consultant of: AbbVie, BMS, Gilead, Galvani, GSK, Janssen, Eli Lilly, Novartis, Pfizer, R-Pharm, UCB