

Background: The preferential Janus kinase-1 inhibitor FIL is approved for treatment of moderate to severe active RA in Europe and Japan.
Objectives: Efficacy and safety of FIL were assessed in pts with IR to MTX who completed a Phase 3 trial (NCT02889796) 1 and enrolled in an LTE (NCT03025308).
Methods: Pts completing the PS 1 on study drug were eligible to enter the LTE (data cutoff: June 1, 2020). Median exposure: 2.2 years (y). Efficacy data to W48 are reported for 4 treatment groups (all with background MTX): pts receiving FIL 200 mg (FIL200) or FIL 100 mg (FIL100) in the PS and continuing their dose in LTE (FIL200/FIL200, FIL100/FIL100) and ADA pts rerandomized, double blind, to FIL200 or FIL100 for LTE (ADA/FIL200, ADA/FIL100); safety data are reported.
Results: As of June 1, 2020, 522/571 (91%) FIL200/FIL200, 502/570 (88%) FIL100/FIL100, 118/128 (92%) ADA/FIL200, and 115/130 (89%) ADA/FIL100 pts remained on study drug. LTE baseline disease characteristics were similar between groups: mean duration of RA approximately 8.7 y; DAS28(CRP) 2.55, and mean concurrent MTX dosage was 15.0 mg/week. Proportions of pts achieving ACR20/50/70, DAS28(CRP) ≤3.2, <2.6, and CDAI ≤10, ≤2.8 were generally maintained in all LTE groups through W48 (
EAIRs of TEAEs in LTE, as of June 1, 2020
| 1TEAE, n (%) | 3FIL200+MTX → FIL200+MTX | 6ADA+MTX → FIL200+MTX | 9FIL100+MTX → FIL100+MTX | 12ADA+MTX → FIL100+MTX |
|---|---|---|---|---|
| 2EAIR (95% CI) | 4n=571 | 7n=128 | 10n=570 | 13n=130 |
| 5PYE=859.4 | 8PYE=197.8 | 11PYE=852.3 | 14PYE=192.6 | |
| TEAE | 429 (75.1) | 91 (71.1) | 443 (77.7) | 88 (67.7) |
| 49.9 (45.4, 54.9) | 46.0 (37.5, 56.5) | 52.0 (47.4, 57.0) | 45.7 (37.1, 56.3) | |
| TEAE Grade ≥3 | 64 (11.2) | 15 (11.7) | 72 (12.6) | 7 (5.4) |
| 7.4 (5.8, 9.5) | 7.6 (4.6, 12.6) | 8.4 (6.7, 10.6) | 3.6 (1.7, 7.6) | |
| TE serious AE | 52 (9.1) | 13 (10.2) | 60 (10.5) | 9 (6.9) |
| 6.1 (4.6, 7.9) | 6.6 (3.8, 11.3) | 7.0 (5.5, 9.1) | 4.7 (2.4, 9.0) | |
| Death | 3 (0.5) | 2 (1.6) | 3 (0.5) | 2 (1.5) |
| 0.3 (0.1, 1.1) | 1.0 (0.3, 4.0) | 0.4 (0.1, 1.1) | 1.0 (0.3, 4.2) | |
| TE infections | 243 (42.6) | 52 (40.6) | 249 (43.7) | 43 (33.1) |
| 28.3 (24.9, 32.1) | 26.3 (20.0, 34.5) | 29.2 (25.8, 33.1) | 22.3 (16.6, 30.1) | |
| TE serious infections | 7 (1.2) | 2 (1.6) | 13 (2.3) | 1 (0.8) |
| 0.8 (0.4, 1.7) | 1.0 (0.3, 4.0) | 1.5 (0.9, 2.6) | 0.5 (0.1, 3.7) | |
| Opportunistic infections | 2 (0.4) | 0 | 2 (0.4) | 0 |
| 0.2 (0, 0.8) | 0 (0, 1.9) | 0.2 (0, 0.8) | 0 (0, 1.9) | |
| TE herpes zoster | 16 (2.8) | 5 (3.9) | 13 (2.3) | 1 (0.8) |
| 1.9 (1.1, 3.0) | 2.5 (1.1,6.1) | 1.5 (0.9, 2.6) | 0.5 (0.1, 3.7) | |
| TE MACE (adjudicated) | 1 (0.2) | 0 | 3 (0.5) | 3 (2.3) |
| 01 (0, 0.6) | 0 (0, 1.9) | 0.4 (0.1, 1.1) | 1.6 (0.5, 4.8) | |
| TE DVT/PE (adjudicated) | 3 (0.5) | 0 | 3 (0.5) | 0 |
| 0.3 (0.1, 1.0) | 0 (0, 1.9) | 0.4 (0.1, 1.0) | 0 (0, 1.9) | |
| Malignancies (excluding NMSC) | 5 (0.9) | 3 (2.3) | 4 (0.7) | 0 |
| 0.6 (0.2, 1.4) | 1.5 (0.5, 4.7) | 0.5 (0.1, 1.2) | 0 (0, 1.9) | |
| NMSC | 3 (0.5) | 0 | 2 (0.4) | 0 |
| 0.3 (0.1, 1.0) | 0 (0, 1.9) | 0.2 (0, 0.8) | 0 (0, 1.9) |
DVT, deep vein thrombosis; MACE, major adverse cardiovascular event; NMSC, nonmelanoma skin cancer; PE, pulmonary embolism; TE, treatment-emergent
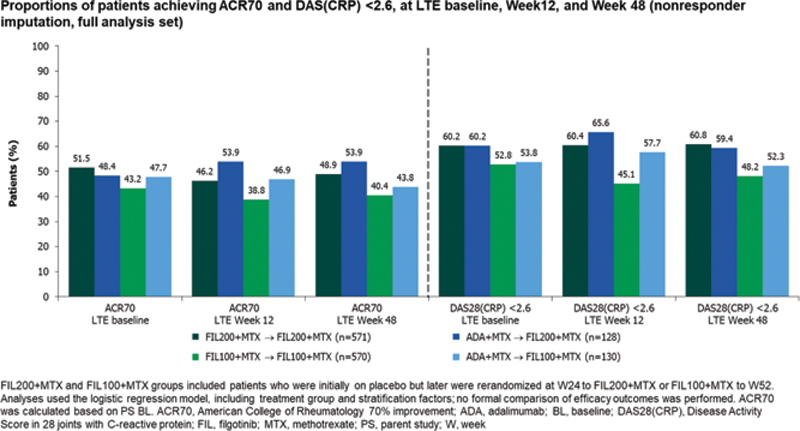
Conclusion: During the LTE through W48, response rates generally were maintained for FIL/FIL and ADA/FIL pts. Though there were differences between LTE groups, safety was largely comparable and consistent with PS observations 1 and previously reported results from 7 trials 2 : rates of AEs of special interest were low; all confidence intervals were overlapping. Limitation: the LTE was not formally randomized for comparison between FIL/FIL and ADA/FIL treatment groups, the groups were of unequal size, and the switch from ADA to FIL for LTE was by design, rather than based on disease activity.
REFERENCES:
[1]Combe B et al. Ann Rheum Dis 2021;80:848–58.
[2]Winthrop K et al. Arthritis Rheumatol 2020;72(suppl 10); abstract 0229.
Acknowledgements: This study was funded by Gilead Sciences, Inc., Foster City, CA. Medical writing support was provided by Claudine Bitel, PhD, of AlphaScientia, LLC, San Francisco, CA; and funded by Gilead Sciences, Inc., Foster City, CA.
Disclosure of Interests: Bernard Combe Speakers bureau: BMS, Eli Lilly & Co., Gilead Sciences, Inc., MSD, Pfizer, Roche-Chugai, and UCB, Consultant of: AbbVie, Eli Lilly & Co., Gilead Sciences, Inc., Janssen, Pfizer, Roche-Chugai, and Sanofi, Grant/research support from: Novartis, Pfizer, and Roche-Chugai, Yoshiya Tanaka Speakers bureau: AbbVie, Asahi-Kasei, Astellas, Bristol-Myers, Chugai, Daiichi- Sankyo, Eli Lilly, Eisai, Gilead, GSK, Janssen, Mitsubishi-Tanabe, Novartis, Pfizer, Sanofi, and YL Biologics, Consultant of: AbbVie, Ayumi, Daiichi- Sankyo, Eli Lilly, GSK, Sanofi, and Taisho, Grant/research support from: AbbVie, Asahi-Kasei, Chugai, Daiichi-Sankyo, Eisai, Mitsubishi-Tanabe, and Takeda, Paul Emery Consultant of: AbbVie, BMS, Celltrion, Gilead, Lilly, Novartis, Roche, Samsung, and Sandoz, Grant/research support from: AbbVie, BMS, Lilly, and Samsung, Alena Pechonkina Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., Albert Kuo Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., Qi Gong Shareholder of: Gilead Sciences, Inc., Employee of: Gilead Sciences, Inc., Katrien Van Beneden Shareholder of: Galapagos NV, Employee of: Galapagos NV, Vijay Rajendran Shareholder of: Galapagos NV, Employee of: Galapagos NV, Hendrik Schulze-Koops Speakers bureau: AbbVie, Amgen, BMS, Celgene, Celltrion, Chugai, Gilead, Janssen, Eli Lilly and Company, Merck Sharp & Dohme, Novartis-Sandoz, Pfizer, Roche, and Sanofi, Consultant of: AbbVie, Amgen, BMS, Celgene, Celltrion, Chugai, Gilead, Janssen, Eli Lilly and Company, Merck Sharp & Dohme, Novartis-Sandoz, Pfizer, Roche, and Sanofi, Grant/research support from: AbbVie and Novartis