

Background: Upadacitinib (UPA) was previously evaluated in two Phase 2, randomized, controlled trials (RCTs) in patients (pts) with rheumatoid arthritis (RA) and inadequate response to tumor necrosis factor inhibitors (BALANCE-1) or methotrexate (BALANCE-2).
Objectives: To assess the final safety and efficacy of UPA in BALANCE-EXTEND, a 312-week open-label extension (OLE) enrolling pts who completed either BALANCE-1 or BALANCE-2.
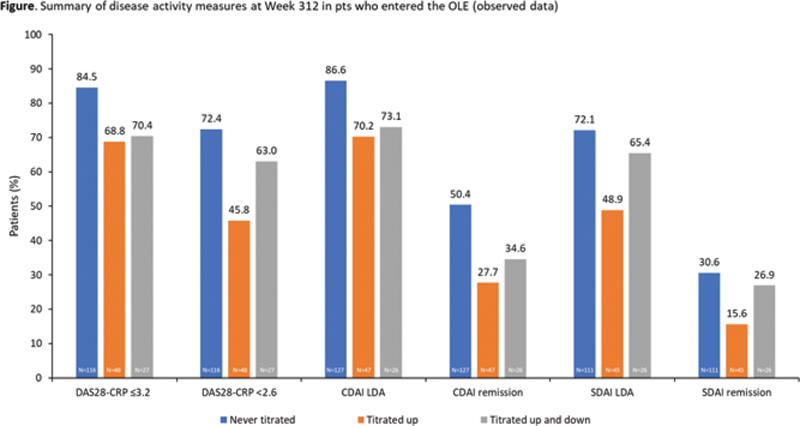
Methods: All pts initially received UPA 6 mg twice daily (BID). Increase to 12 mg BID was required for pts with <20% improvement in swollen or tender joint counts (S/TJC) at Week 6 or 12, and permitted for those not achieving Clinical Disease Activity Index (CDAI) low disease activity (LDA). Pts with <20% improvement in SJC or TJC 6 weeks after escalation, or at any two consecutive visits, discontinued. Return to 6 mg BID was permitted for safety or tolerability reasons. After January 2017, the 6 and 12 mg BID doses were replaced by 15 and 30 mg once-daily (QD) extended-release equivalents. As-observed efficacy data are shown at Week 312 for three subgroups: pts who received 6 mg BID/15 mg QD throughout (“Never titrated”), those titrated up to 12 mg BID/30 mg QD for efficacy (“Titrated up”), and those titrated up to 12 BID/30 mg QD and then back to 6 mg BID/15 mg QD due to safety concerns (“Titrated up and down”). Exposure-adjusted adverse events (EAERs) per 100 patient-years (PY) of exposure were summarized from OLE Day 1 in all pts who received UPA (Any UPA).
Results: Overall, 493 pts entered the OLE, receiving UPA for ≤6.2 years (Never titrated, n=306; Titrated up, n=149; Titrated up and down, n=38), and 270 pts (54.8%) discontinued, mostly due to withdrawal of consent (16.8%; n=83) or AEs (14.6%; n=72). Mean (standard deviation) duration of UPA exposure was 3.8 (2.4) years (range <1–6.2 years); cumulative exposure was 1863 PY. The AE profile in pts receiving UPA 15 mg was generally similar to the Any UPA population, and to that observed in the Phase 3 UPA 15 mg clinical trial population (
Summary of AEs in pts who received UPA 6 mg BID/15 mg QD in the OLE and in the UPA 15 mg Phase 3 study program
| BALANCE-EXTEND (UPA 6 mg BID/15 mg QD) | UPA 15 mg – Phase 3 program b | |
|---|---|---|
| Events/100 PY (95 CI) a | Events/100 PY (95% CI) a | |
| N=493, PY=1277 | N=3209, PY=9079 | |
| Any AE | 138.4 (132.0, 145.0) | 205.5 (202.5, 208.5) |
| Any SAE | 7.9 (6.4, 9.6) | 12.4 (11.7, 13.2) |
| AE leading to discontinuation | 4.2 (3.2, 5.5) | 4.9 (4.4, 5.3) |
| Death | 0.4 (0.1, 0.9) | 0.5 (0.4, 0.7) c |
| Infection | 49.2 (45.5, 53.2) | 63.9 (62.3, 65.6) |
| Serious infection | 1.4 (0.8, 2.2) | 2.8 (2.4, 3.1) |
| Opportunistic infection | 0.2 (0.0, 0.6) | 0.3 (0.2, 0.4) |
| Herpes zoster | 2.0 (1.3, 3.0) | 3.0 (2.6, 3.3) |
| Anemia | 1.1 (0.6, 1.8) | 3.0 (2.7, 3.4) |
| Neutropenia | 1.3 (0.8, 2.1) | 2.1 (1.8, 2.5) |
| Lymphopenia | 1.7 (1.1, 2.6) | 1.7 (1.4, 1.9) |
| Gastrointestinal perforation | 0 | <0.1 (0.0, 0.1) |
| Any malignancy | 1.2 (0.7, 1.9) | 1.1 (0.9, 1.4) |
| Non-melanoma skin cancer (NMSC) | 0.4 (0.1, 0.9) | 0.4 (0.3, 0.5) |
| Excluding NMSC | 0.8 (0.4, 1.4) | 0.7 (0.6, 0.9) |
| Creatinine phosphokinase elevation | 3.4 (2.5, 4.6) | 4.4 (4.0, 4.9) |
| Hepatic disorder | 4.1 (3.0, 5.3) | 10.2 (9.5, 10.8) |
| Venous thromboembolism | 0.5 (0.2, 1.0) | 0.4 (0.3, 0.6) |
| Major adverse cardiovascular event | 0.5 (0.2, 1.0) | 0.4 (0.3, 0.5) |
a Multiple events occurring in the same pts are counted in the calculation of events/100 PY. b Cut-off, June 30, 2021. c Based on 9080 PY.
Conclusion: In this OLE, UPA treatment over ~312 weeks showed sustained long-term efficacy in pts with RA who had completed Phase 2 RCTs. Overall safety results showed that UPA was well tolerated over time; the types and frequencies of AEs were consistent with those in pts with similar populations of moderately to severely active RA receiving Janus kinase inhibitors.
Acknowledgements: AbbVie funded this study; contributed to its design; participated in data collection, analysis, and interpretation of the data; and participated in the writing, review, and approval of the abstract. AbbVie and the authors thank all study investigators for their contributions and the patients who participated in this study. No honoraria or payments were made for authorship. Medical writing support was provided by Dan Booth, PhD, of 2 the Nth (Cheshire, UK), and was funded by AbbVie.
Disclosure of Interests: Alan Kivitz Shareholder of: Amgen, Gilead, GlaxoSmithKline, Novartis, Pfizer, and Sanofi (stocks or options), Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, Flexion, Genzyme, Gilead, Horizon, Janssen, Merck, Novartis, Pfizer, Regeneron, Sanofi, Sanofi Aventis, SUN Pharma Advanced Research, and UCB, Grant/research support from: AbbVie (his institution received fees for his role as a Principal Investigator in the study), Alvin F. Wells Consultant of: AbbVie, Juan Ignacio Vargas Consultant of: AbbVie, Grant/research support from: AbbVie (as a Principal Investigator in the study), Herbert S.B. Baraf Consultant of: Gilead and Janssen, Grant/research support from: AbbVie, Eli Lilly, Genentech, Gilead, and Janssen, Maureen Rischmueller Consultant of: AbbVie, Bristol-Myers Squibb, CSL Behring, Eli Lilly, Gilead Sciences, Janssen Global Services, Pfizer, Sanofi US Services, and UCB Biosciences, Grant/research support from: AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Janssen Global Services, Novartis, Pfizer, Sanofi Pasteur Biologics, and UCB Biosciences, Justin Klaff Shareholder of: AbbVie (may own stocks or options), Employee of: AbbVie, Nasser Khan Shareholder of: AbbVie (may own stocks or options), Employee of: AbbVie, Yihan Li Shareholder of: AbbVie (may own stocks or options), Employee of: AbbVie, Kyle Carter Shareholder of: AbbVie (may own stocks or options), Employee of: AbbVie, Alan Friedman Shareholder of: AbbVie (may own stocks or options), Employee of: AbbVie, Patrick Durez Speakers bureau: Eli Lilly and Galapagos