

Background: The efficacy of upadacitinib (UPA), an oral Janus kinase inhibitor (JAKi), in the treatment of rheumatoid arthritis (RA) has been demonstrated in the phase 3 SELECT clinical trial program. 1–6 However, few real-world data have been reported to date.
Objectives: To assess the 6-month effectiveness of UPA in patients (pts) with RA initiating UPA treatment in clinical practice.
Methods: This observational study included US-based pts from the United Rheumatology Normalized Integrated Community Evidence (UR-NICE) database who initiated UPA 15 mg once daily from Aug 2019 to the data cut-off in Nov 2021. Pts with ≥6 months of baseline (BL) data before UPA initiation, and with Clinical Disease Activity Index (CDAI) score recorded at BL and 6 months (±45 days) after initiation, were included in the analysis. Effectiveness measures included CDAI score, Routine Assessment of Patient Index Data 3 (RAPID3), and Disease Activity Score in 28 joints based on C-reactive protein (DAS28-CRP); patient-reported outcomes (PROs) including Health Assessment Questionnaire-Disability Index (HAQ-DI), Pain, and Patient’s Global Assessment of Disease Activity (PtGA); and Physician’s Global Assessment of Disease Activity (PhGA). Subgroup analyses were conducted by prior tumor necrosis factor inhibitor (TNFi) and tofacitinib (TOFA) treatment history.
Results: 363 pts were included in the analysis and most were female (80.2%) (
Demographic and baseline characteristics
| Parameter, n (%) | Full analysis set | Prior TNFi | Prior TOFA | |||
|---|---|---|---|---|---|---|
| (N=363) | (n=199) | (n=143) | ||||
| Female | 291 (80.2) | 156 (78.4) | 119 (83.2) | |||
| Age, years | ||||||
| <40 | 22 (6.1) | 11 (5.5) | 8 (5.6) | |||
| 40–<65 | 240 (66.1) | 132 (66.3) | 94 (65.7) | |||
| ≥65 | 101 (27.8) | 56 (28.1) | 41 (28.7) | |||
| Oral steroid use | 185 (51.0) | 103 (51.8) | 83 (58.0) | |||
| Parameter, mean (SD ) | N | Mean (SD ) | n | Mean (SD ) | n | Mean (SD ) |
| Duration of RA, years | 276 | 4.5 (3.1) | 162 | 5.1 (3.0) | 113 | 5.1 (2.9) |
| Body mass index, kg/m 2 | 321 | 30.0 (6.9) | 175 | 29.9 (6.6) | 125 | 29.2 (6.7) |
| Oral steroid dose (prednisone equivalent), mg/day | 154 | 7.9 (6.9) | 87 | 7.8 (6.5) | 70 | 7.6 (6.1) |
| Methotrexate dose, mg/week | 119 | 18.3 (4.9) | 74 | 17.8 (5.2) | 37 | 18.2 (4.7) |
| C-reactive protein, mg/L | 228 | 9.6 (16.2) | 132 | 9.4 (15.0) | 90 | 11.3 (17.9) |
| CDAI | 363 | 21.2 (12.8) | 199 | 22.1 (13.0) | 143 | 21.7 (13.3) |
| RAPID3 | 268 | 4.7 (2.1) | 141 | 4.7 (2.2) | 100 | 4.9 (2.1) |
| DAS28-CRP | 228 | 3.9 (1.3) | 132 | 4.0 (1.4) | 90 | 4.2 (1.3) |
| HAQ-DI a | 273 | 2.6 (2.1) | 148 | 2.8 (2.2) | 106 | 3.0 (2.2) |
| Pain b | 338 | 59.6 (26.6) | 186 | 58.4 (27.2) | 131 | 61.3 (25.1) |
| PtGA b | 363 | 54.1 (25.6) | 199 | 54.7 (26.9) | 143 | 55.9 (25.2) |
| PhGA b | 363 | 41.3 (26.0) | 199 | 41.2 (24.8) | 143 | 40.7 (26.8) |
a 0–10 visual analog scale. b 0–100 visual analog scale. SD, standard deviation.
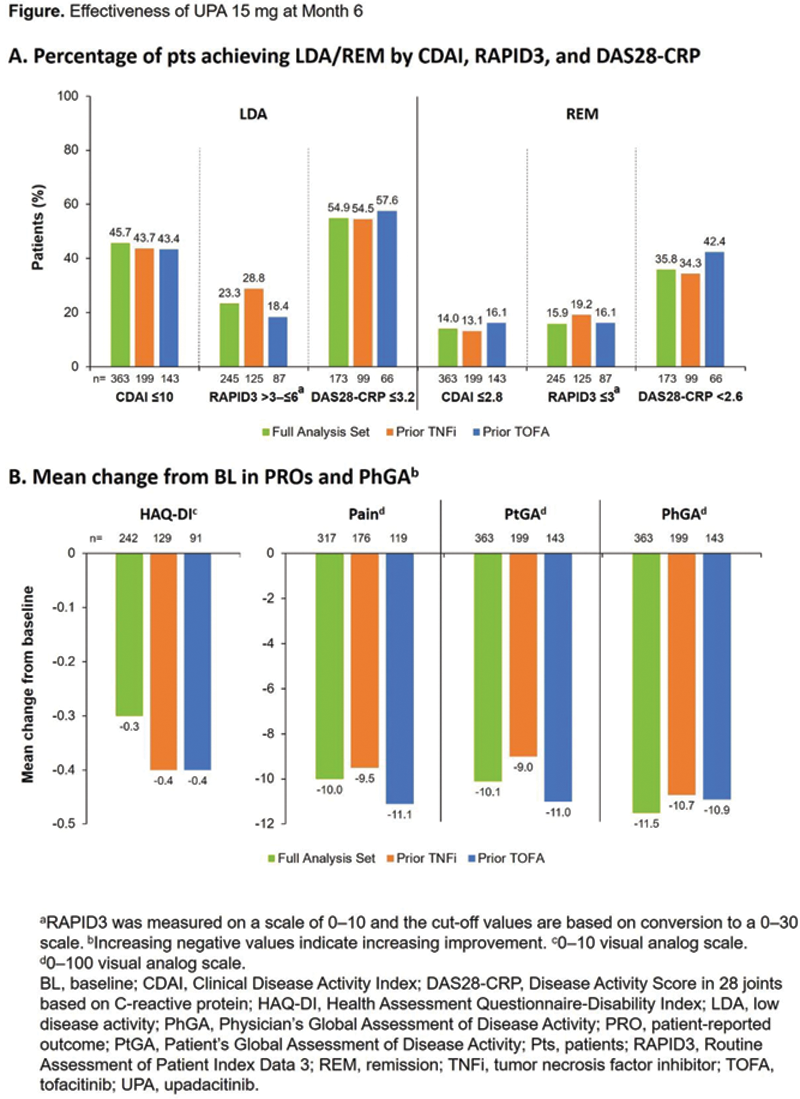
Conclusion: In this study, almost half (46%) of pts treated with UPA achieved CDAI LDA at 6 months and 14% achieved CDAI REM. Improvements in all PROs and PhGA were observed. Effectiveness of UPA was not impacted by prior TNFi or TOFA exposure, supporting UPA as an effective treatment option in clinical practice, including in pts with prior exposure to advanced therapy.
REFERENCES:
[1]Burmester GR, et al. Lancet 2018;391:2503–12.
[2]Smolen JS, et al. Lancet 2019;393:2303–11.
[3]Fleischmann R, et al. Arthritis Rheumatol 2019;71:1788–800.
[4]Genovese MC, et al. Lancet 2018;391:2513–24.
[5]van Vollenhoven R, et al. Arthritis Rheumatol 2020;72:1607–20.
[6]Rubbert-Roth A, et al. N Engl J Med 2020;383:1511–21.
Acknowledgements: AbbVie funded this study; contributed to its design; participated in data collection, analysis, and interpretation of the data; and participated in the writing, review, and approval of the abstract. AbbVie and the authors thank all study investigators for their contributions and the patients who participated in this study. No honoraria or payments were made for authorship. Medical writing support was provided by Laura Chalmers, PhD, of 2 the Nth (Cheshire, UK), and was funded by AbbVie.
Disclosure of Interests: Allan Gibofsky Shareholder of: AbbVie, Amgen, Johnson & Johnson, and Pfizer (stocks), Consultant of: AbbVie, Celgene, Eli Lilly, Flexion, Pfizer, Relburn Pharma, and Samumed (consulting fees); and Gerson Lehrman Group (paid consultant with investment analysts), Mark E. Pearson Shareholder of: AbbVie (may own stock or options), Andrew Concoff Speakers bureau: Flexion Therapeutics and Exagen, Consultant of: Flexion Therapeutics and Exagen, Anna Shmagel Shareholder of: AbbVie (may own stock or options), Employee of: AbbVie, Patrick Zueger Shareholder of: AbbVie (may own stock or options), Employee of: AbbVie, Yanna Song Shareholder of: AbbVie (may own stock or options), Employee of: AbbVie, Lauren Smith Shareholder of: AbbVie (may own stock or options), Employee of: AbbVie, Grace C. Wright Speakers bureau: AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Exagen, Myriad Autoimmune, Novartis, Sanofi/Regeneron, UCB, and Vindico, Consultant of: AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Exagen, Gilead, Janssen, Myriad Autoimmune, Novartis, Pfizer, Sanofi/Regeneron, and UCB, Employee of: Association of Women in Rheumatology (President and Founder)