

Background: Incomplete SLE disease control is associated with progressive organ damage, poor quality of life, and increased mortality. 1–3 Sustained reduction in overall disease activity is therefore an important treatment goal.
Objectives: To investigate sustained British Isles Lupus Assessment Group 2004–based Composite Lupus Assessment (BICLA) response and British Isles Lupus Assessment Group (BILAG) responses by organ domain in pooled data from the TULIP-1 and TULIP-2 trials of the type I interferon receptor monoclonal antibody, anifrolumab, in patients with SLE. 4,5
Methods: TULIP-1 (NCT02446912) and TULIP-2 (NCT02446899) were phase 3, randomized, placebo-controlled, 52-week trials of intravenous anifrolumab administered every 4 weeks for 48 weeks in eligible patients with moderate to severe SLE who were receiving standard therapy. 4,5 Sustained BICLA and BILAG response rates, measured as the number of consecutive patient visits with BICLA or BILAG domain responses, respectively, from Week 4 to Week 52 were compared between the anifrolumab vs placebo groups. BILAG-2004 response was defined as a reduction from A (severe disease) at baseline to B (moderate), C (mild), or D (no current disease) or reduction from B at baseline to C or D. 6
Results: In total, 360 patients received anifrolumab 300 mg and 366 patients received placebo in the TULIP-1 and TULIP-2 trials. Analysis of pooled TULIP data revealed that more patients who received anifrolumab had sustained BICLA responses compared with placebo (
Number of Patients With Consecutive Visits of Sustained BICLA Response Up to and Including Week 52 in Pooled TULIP-1 and TULIP-2 Trials
| n (%) | Anifrolumab 300 mg | Placebo |
|---|---|---|
| (n=360) | (n=366) | |
| ≥3 months | 121 (33.6) | 75 (20.5) |
| (≥5 visits, Week 36–52 ) | ||
| ≥6 months | 98 (27.2) | 55 (15.0) |
| (≥8 visits, Week 24–52 ) | ||
| ≥9 months | 59 (16.4) | 31 (8.5) |
| (11 visits, Week 12–52 ) | ||
| 12 months | 33 (9.2) | 7 (1.9) |
| (13 visits, Week 4–52 ) |
BICLA, British Isles Lupus Assessment Group–based Composite Lupus Assessment.
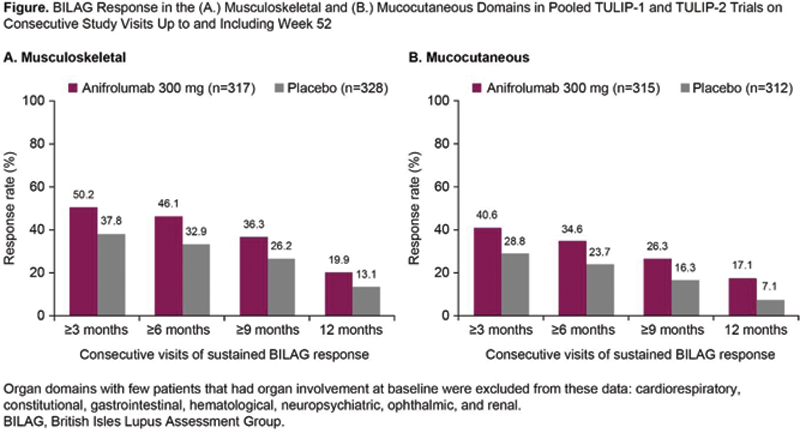
Conclusion: In two phase 3 studies, more anifrolumab-treated patients achieved sustained BICLA and BILAG responses compared with placebo. In the frequently affected musculoskeletal and mucocutaneous domains, sustained treatment benefit of anifrolumab over placebo was observed. These data support the durable clinical benefit of anifrolumab treatment in patients with moderate to severe SLE who are receiving standard therapy.
REFERENCES:
[1]Chambers SA. Rheumatology (Oxford ). 2009;48:673–5.
[2]Murimi-Worstell IB. BMJ Open. 2020;10:e031850.
[3]Olesińska M. Reumatologia . 2018;56:45–54.
[4]Furie RA. Lancet Rheumatol . 2019;1:e208–19.
[5]Morand EF. N Engl J Med . 2020;382:211–21.
[6]Isenberg DA. Rheumatology (Oxford ). 2005;44:902–6.
Acknowledgements: Writing assistance by Victoria Alikhan (Fishawack Health). This study was sponsored by AstraZeneca.
Disclosure of Interests: Ronald van Vollenhoven Speakers bureau: AbbVie, Galapagos, GSK, Janssen, Pfizer, R-Pharma, UCB, Consultant of: AbbVie, AstraZeneca, Biogen, BMS, Galapagos, Janssen, Miltenyi, Pfizer, UCB, Grant/research support from: MSD, Pfizer, Roche, BMS, GSK, UCB, Richard Furie Speakers bureau: AstraZeneca, Genentech, Consultant of: AstraZeneca, Grant/research support from: AstraZeneca, Eric F. Morand Speakers bureau: GSK, Novartis, Paid instructor for: AstraZeneca, Biogen, Eli Lilly, Consultant of: AstraZeneca, Biogen, Bristol Myers Squibb, Eli Lilly, EMD Serono, Genentech, GSK, Janssen, Servier, Grant/research support from: Abbvie, AstraZeneca, Bristol Myers Squibb, GSK, Janssen, Raj Tummala Shareholder of: AstraZeneca, Employee of: AstraZeneca, Emmanuelle Maho Employee of: AstraZeneca, Catharina Lindholm Employee of: AstraZeneca