

Background: Systemic lupus erythematosus (SLE), a severe multiorgan autoimmune disease, involves dysfunction of multiple immune system components. In preclinical studies, KZR-616, a first-in-class small molecule selective inhibitor of the immunoproteasome, demonstrated pleiotropic immunomodulatory functions encompassing both innate and adaptive immune pathways 1,2 . In the recently completed MISSION Phase 1b trial in SLE patients (pts), KZR-616 exhibited encouraging safety, tolerability, and clinical improvements 3 . Preliminary biomarker analysis of the first two cohorts was previously reported 4 . Here, we present complete biomarker results from all cohorts in this study.
Objectives: 1) To characterize biomarker changes (gene expression, circulating protein levels and immune cell phenotypes) following KZR-616 treatment. 2) To evaluate correlations between biomarker changes and treatment response to KZR-616.
Methods: The open-label Phase 1b MISSION Trial (NCT 03393013) was a 25-week trial of KZR- 616 administered subcutaneously once weekly at doses ranging from 30-75 mg for 13 weeks (W) with a 12 W follow-up. Forty-seven SLE pts with and without nephritis were enrolled with 35 completing all 25 W of study. Clinical response definitions at W13 or later were defined as either a >4 reduction of SLEDAI-2K or >50% drop in swollen joint counts (SJC) (baseline SJC>2). Biomarker samples included whole blood in RNA tubes (31 pts), cryopreserved PBMCs (15 pts), plasma (35 pts) and urine (2 pts). Whole blood RNA sequencing was performed using Illumina TruSeq. Differential expression was modelled using DESeq2. Fast pre-ranked gene set enrichment analysis (GSEA) was performed with gene sets derived from published literature. Cryopreserved PBMCs were analysed by flow cytometry to profile immune cell subtypes. Plasma protein was measured by Meso Scale Discovery (MSD) kits. Healthy volunteer (HV, N=12) samples were used to establish potential disease-related biomarker changes in patients at baseline (BL).
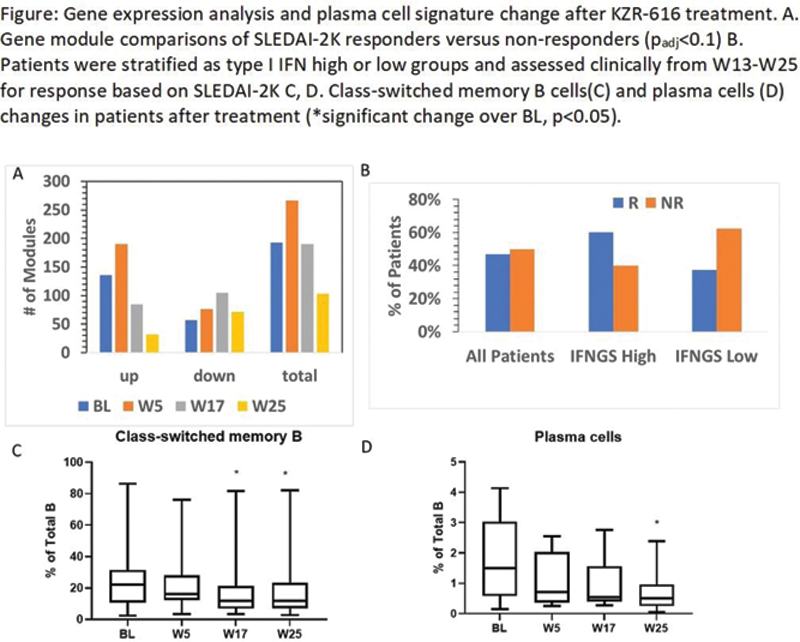
Results: Many gene modules were found to be altered at BL in SLE pts, relative to HV, including those representing interferon (IFN) response and B/plasma cells and changes were consistent with our prior report 4 . Comparisons of SLEDAI-2K responders (R) versus non-responders (NR) revealed enrichment of 193 gene modules (57 down and 136 up-regulated, p adj ,<0.1) at BL and 190 modules (105 down and 85 up) 4 weeks after treatment (W17). Expression of a 4-gene IFN module was enriched in SLEDAI-2K R vs NR at BL; expression of this module trended downward at W17 in R, whereas it increased over time in NR at W 5. Reduced numbers of circulating class-switched memory B cells and IgG-producing plasma cells were observed at W17 and/or W25. Plasma levels of BAFF were consistently increased at W5 but returned to BL levels by W17. Plasma levels of CD169/ SIGLEC-1, a monocyte activation marker, were higher at BL in SJC R vs. NR and were reduced after treatment in both groups. Two pts with active proliferative nephritis were enrolled into this study and both showed a reduction in levels of urine CD163 (uCD163), while plasma levels of this marker were stable.
Conclusion: Our integrative analysis indicates that KZR-616 elicits a potent effect on multiple immune pathways in SLE patients. Potential new biomarkers (gene, gene module, circulating protein) that may be useful for prediction of patient response were identified. Future biomarker studies in placebo-controlled trials may further our understanding of KZR-616 mechanism of action, patient selection and prediction of patient responses.
REFERENCES:
[1]Kirk, C.J. et al., Cells 2022, 11(1):9
[2]Muchamuel T, Arthritis Rheumatol. 2019; 71 (suppl 10).
[3]Furie R et al., Annals of the Rheumatic Diseases 2021;80:595-596,
[4]Fan R, et al., Arthritis Rheumatol. 2019; 71 (suppl 10).
Acknowledgements: Kezar Life Sciences acknowledges the support of site investigators and patient participants in the MISSION study.
Disclosure of Interests: Andrea Fan Shareholder of: Kezar, Employee of: Kezar, Brian Tuch Consultant of: Kezar, Tony Muchamuel Shareholder of: Kezar, Employee of: Kezar, Janet Anderl Shareholder of: Kezar, Employee of: Kezar, Richard Leff Consultant of: Kezar, Noreen Henig Shareholder of: Kezar, Employee of: Kezar, Christopher Kirk Shareholder of: Kezar, Employee of: Kezar