

Background: Large vessel involvement in Giant Cell Arteritis (GCA), especially the aorta and/or its main branches, is frequent. Tocilizumab (TCZ) has shown efficacy and safety in GCA and other large-vessel vasculitis (1-4 ).
Objectives: To assess the efficacy and safety of TCZ in GCA patients with involvement of the aorta and/or its main branches.
Methods: Multicenter observational study of 196 patients with GCA and involvement of the aorta and/or its major branches treated with TCZ. GCA was diagnosed by: a ) ACR criteria, and/or b ) temporal artery biopsy, and/or c ) imaging techniques. The presence of aortitis was performed by imaging techniques, mainly PET, and A-MRI.
Maintained remission was considered according to EULAR definitions (5 ).
Results: The main features of the 196 patients are showed in
Main features of 196 GCA patients with involvement of the aorta and/or its main branches treated with TCZ.
| GCA (n=196 ) | |
|---|---|
| Features at TCZ onset | |
| Age(years), mean±SD | 71.3±9.5 |
| Sex, female/male (% female) | 148/48 (75) |
| Time from GCA diagnosis to TCZ onset (months), median [IQR] | 7 [2-18.25] |
| Systemic manifestations, n (% ) | |
| Fever, n (%) | 24 (12) |
| Constitutional syndrome, n (%) | 87 (44) |
| PmR, n (%) | 131 (67) |
| Ischaemic manifestations, n (% ) | |
| Visual involvement, n (%) | 16 (8) |
| Headache, n (%) | 74 (38) |
| Jaw claudication, n (%) | 27 (14) |
| Laboratory data | |
| ESR, mm 1st hour, median [IQR] | 32 [14-54] |
| CRP, mg/dL, median [IQR] | 1.5 [0.6-3.2] |
| Prednisone dose, mg/day, median [IQR] | 15 [10-30] |
| Safety after TCZ onset | |
| Relevant adverse events, per 100 patients-year | 12 |
| Serious infections, per 100 patients-year | 4.8 |
A ) Sustained remission, and B ) median prednisone dose required in GCA patients with aortitis treated with tocilizumab
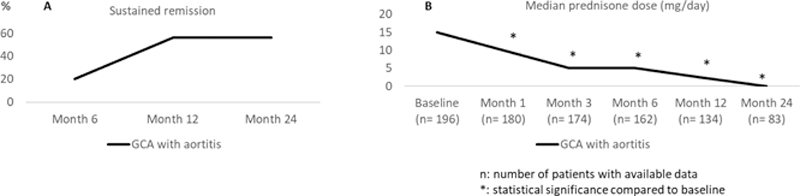
Conclusion: TCZ seems to be effective and relatively safe in GCA patients with involvement of the aorta and/or its main branches.
REFERENCES:
[1]Calderón-Goercke M, et al. Semin Arthritis Rheum. 2019; 49: 126-135. PMID: 30655091
[2]Loricera J, et al. Clin Exp Rheumatol. 2016; 34: S44-53. PMID: 27050507
[ 3]Loricera J, et al. Clin Exp Rheumatol. 2015; 33: S19-31. PMID: 25437450
[4]Prieto-Peña D, et al. Ther Adv Musculoskelet Dis. 2021; 13: 1759720X211020917. PMID: 34211589
[5]Hellmich B, et al. Ann Rheum Dis. 2020; 79: 19-30. PMID: 31270110
Disclosure of Interests: Lara Sanchez-Bilbao: None declared, Javier Loricera Speakers bureau: from Roche, Novartis, UCB Pharma, Celgene, and Grünenthal., Rafael Melero: None declared, Santos Castañeda Speakers bureau: UAM-Roche, EPID- Future chair, Department of Medicine, Universidad Autónoma de Madrid, Madrid, Spain., Clara Moriano: None declared, Iván Ferraz-Amaro: None declared, J. Narváez: None declared, Vicente Aldasoro: None declared, Olga Maiz: None declared, Ignacio Villa-Blanco: None declared, Paloma Vela-Casasempere: None declared, Susana Romero-Yuste: None declared, Jose Luis Callejas-Rubio: None declared, Eugenio de Miguel: None declared, E. Galíndez-Agirregoikoa: None declared, Francisca Sivera: None declared, Carlos Fernández-López: None declared, Carles Galisteo: None declared, Julio Sanchez-Martin: None declared, Monica Calderón-Goercke: None declared, J. Luis Hernández: None declared, Miguel A González-Gay Speakers bureau: Abbvie, Pfizer, Roche, Sanofi, Lilly, Celgene, and MSD., Grant/research support from: AbbVie, MSD, Jansen, and Roche,, Ricardo Blanco Speakers bureau: Abbvie, Pfizer, Roche, Bristol-Myers, Lilly, Janssen, and MSD., Grant/research support from: Abbvie, MSD, and Roche