

Background: Giant cell arteritis (GCA) has the tendency to relapse once treatment is tapered or stopped. Such relapses represent a potential threat to GCA patients as they can lead to severe symptoms and organ damage.
Objectives: To assess the frequency and type of relapses in patients with GCA
Methods: The Real-Life Treatment and Safety (REATS)-GCA cohort has been established by extracting the data on clinical presentation, inflammatory markers, imaging, comorbidities, treatments and serious adverse events of GCA patients from 6 specialized centres in Germany. We undertook descriptive and survival analyses (Kaplan-Meier), and compared baseline characteristics of participants with vs. without relapse. Ethical approval for the cohort was obtained.
Results: We included 395 patients with a mean age of 71 years, including 264 (66.8 %) females and 129 (32.7%) males. Diagnosis of GCA was supported by temporal artery ultrasound in 37%,
18
F-FDG-PET/CT in 29%, temporal artery biopsy in 14% of patients and by MRI or clinically in the remaining patients. 31% of patients presented with an isolated cranial manifestation and 18% with isolated extracranial manifestations. Most common presenting symptoms were headache (57%), fatigue (55%), weight loss (42%) and polymyalgia (38%) (
| Symptom at disease onset | N=395 (%) | Symptom at relapse | N=97 (%) |
|---|---|---|---|
| Headache | 216 (54.7) | Headache | 35 (30.7) |
| Fatigue | 208 (52.7) | Polymyalgia (PMR) | 23 (20.2) |
| Weight loss | 159 (40.3) | Fatigue | 19 (16.7) |
| Polymyalgia (PMR) | 144 (36.5) | Vision impairment | 13 (11.4) |
| Night sweats | 140 (35.4) | Night sweats | 12 (10.5) |
| Headache in the temple area | 125 (31.6) | Headache in the temple area | 12 (10.5) |
| Jaw pain | 121 (30.6) | Jaw pain | 11 (9.6) |
| Vision impairment | 118 (29.9) | Morning stiffness | 7 (6.1) |
| Morning stiffness | 89 (22.5) | Weight loss | 7 (6.1) |
| Fever | 80 (20.3) | Claudication upper limb | 6 (5.3) |
| Swelling temporal arteries | 77 (19.5) | Arthralgia | 6 (5.3) |
| Vision loss | 57 (14.4) | Claudication lower limb | 5 (4.4) |
| Scalp tenderness | 38 (9.6) | Vision loss | 3 (2.6) |
| Claudication upper limb | 38 (9.6) | Arthritis | 3 (2.6) |
| Claudication lower limb | 34 (8.6) | Scalp tenderness | 2 (1.8) |
| Arthralgia | 28 (7.1) | Fever | 2 (1.8) |
| Arthritis | 3 (0.8) | Swelling temporal arteries | 2 (1.8) |
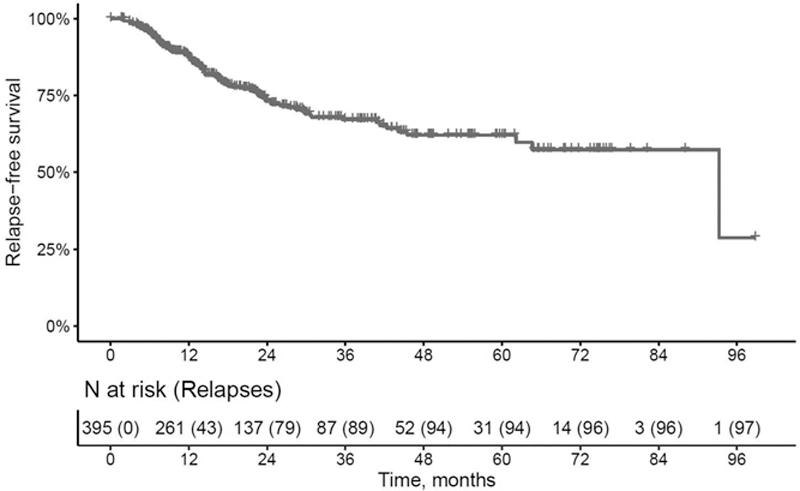
Conclusion: About one fourth of GCA patients relapsed and the overwhelming majority of relapses occurred before patients were able to stop glucocorticoids. The leading symptoms at relapse are headache and fatigue, while loss of vision is rare (0.76%). Baseline characteristics seem to be poorly informative about the risk of relapse, therefore regular monitoring of GCA patients is necessary.
Acknowledgements: This research was financially supported by Roche Pharma Ag and Chugai Pharma Europe Ltd.
Disclosure of Interests: Verena Schönau Speakers bureau: Novartis, Janssen, Grant/research support from: Roche, Chugai, Giulia Corte: None declared, Sebastian Ott: None declared, Koray Tascilar: None declared, Fabian Hartmann: None declared, Bernhard Manger: None declared, Bernhard Hellmich: None declared, Alexander Pfeil: None declared, Peter Oelzner: None declared, Wolfgang A. Schmidt: None declared, Andreas Krause: None declared, Marc Schmalzing: None declared, Matthias Fröhlich: None declared, Michael Gernert: None declared, Nils Venhoff: None declared, Jörg Henes: None declared, Jürgen Rech Speakers bureau: Abbvie, Biogen, BMS, Chugai, GSK, Lilly, MSD; Novartis, Roche, Sanofi, Sobi, UCB,, Consultant of: Biogen, BMS, Chugai, GSK, Lilly, MSD, Novartis, Roche, Sanofi, Sobi, UCB, Grant/research support from: Sobi, Novartis, Georg Schett: None declared