

Background: Patients with eosinophilic granulomatosis with polyangiitis (EGPA) can present with vasculitic or eosinophilic phenotypes. 1 The Phase III MIRRA study demonstrated that patients with EGPA spent more time in remission and had reduced oral corticosteroid (OCS) use with mepolizumab versus placebo. 2
Objectives: To evaluate the efficacy of mepolizumab in patients with a vasculitic EGPA phenotype enrolled in the MIRRA study.
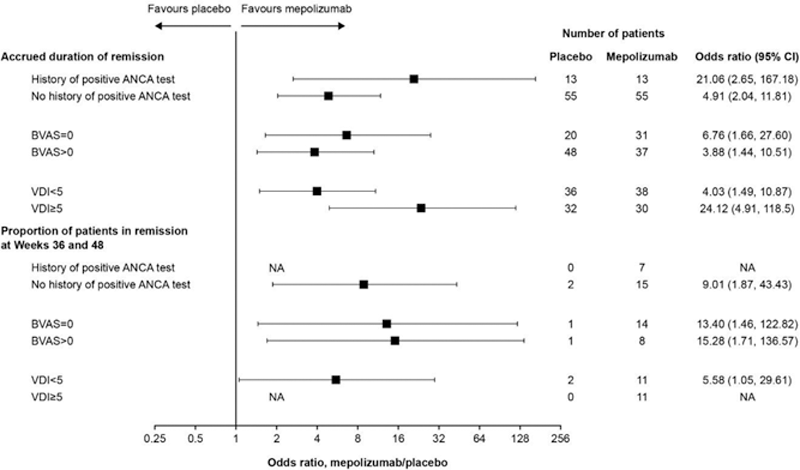
Methods: MIRRA was a Phase III, multicentre, double-blind, parallel-group trial in adult patients with relapsing/refractory EGPA and ≥4 weeks stable OCS treatment. Patients were randomised to receive standard of care plus mepolizumab (300 mg subcutaneously every 4 weeks) or placebo for 52 weeks. Primary endpoints were: accrued weeks of remission (defined as Birmingham Vasculitis Activity Score [BVAS] of 0 and OCS dose ≤4 mg/day prednisolone or equivalent) over the 52-week study period categorised in weeks (0, >0 to <12, 12 to <24, 24 to <36 and ≥36 weeks), and the proportion of patients in remission at both Weeks 36 and 48. This post hoc analysis used data from the MIRRA study to evaluate these endpoints according to patients’ antineutrophil cytoplasmic antibody (ANCA) history (current or previous positive test for myeloperoxidase[MPO]/proteinase 3[PR3]-ANCA at study baseline versus no history of a positive MPO/PR3-ANCA test at baseline), baseline BVAS (=0 vs >0) and Vasculitis Damage Index (VDI) score (<5 vs ≥5). Types of disease relapse (vasculitis [BVAS >0], asthma [active asthma symptoms and/or signs with a worsening in Asthma Control Questionnaire-6 score] and sinonasal [active nasal and/or sinus disease with a worsening in ≥1 sinonasal symptom questions]) reported during the treatment period were also described. EGPA disease characteristics focusing on vasculitic components were assessed in patients who did and did not achieve remission at any point during the study.
Results: Of the 136 patients in the study, 26 (19%) had a history of a positive ANCA test at study baseline and 110 (81%) did not. In addition, 51 (38%) had a BVAS =0 at baseline while 85 (63%) had a BVAS >0; 74 (54%) had a VDI <5 at baseline and 62 (46%) had a VDI ≥5. Accrued remission duration was greater with mepolizumab versus placebo, irrespective of ANCA positive status, baseline BVAS or baseline VDI score (
Mepolizumab efficacy by patient baseline characteristics
ANCA, antineutrophil cytoplasmic antibody; BVAS, Birmingham Vasculitis Activity Score; CI, confidence interval; NA, data not available – estimate could not be calculated owing to a lack of patients in the placebo group achieving remission at Weeks 36 and 48; VDI, Vasculitis Damage Index.
Conclusion: Mepolizumab is associated with clinical benefits in patients with EGPA, including those with and without a vasculitic phenotype.
REFERENCES:
[1]Latorre M et al. Eur Respir J. 2013;42(Suppl 57):1797.
[2]Wechsler ME et al. N Engl J Med . 2017;376(20):1921–32.
Acknowledgements: Funding: GSK (MEA115921; NCT02020889); the Division of Intramural Research, NIAID, NIH funded in part time spent on this abstract by one of the authors (PK).
Disclosure of Interests: Benjamin Terrier Consultant of: Roche, Chugai, GSK, AstraZeneca, Bristol Myers Squibb, Terumo BCT, Sanofi, LFB and Grifols, David Jayne Speakers bureau: Amgen Vifor, Consultant of: AstraZeneca, Aurinia, BMS, Boehringer Ingelheim, ChemoCentryx, GSK, Janssen, Novartis, Roche/Genentech, Takeda and Vifor, Grant/research support from: AstraZeneca, Aurinia, BMS, Boehringer Ingelheim, ChemoCentryx, GSK, Janssen, Novartis, Roche/Genentech, Takeda and Vifor, Bernhard Hellmich Speakers bureau: AbbVie, BMS, Boehringer Ingelheim, Chugai, GSK, InflaRx, Novartis, Pfizer, Roche and Vifor Pharma, Consultant of: AbbVie, BMS, Boehringer Ingelheim, Chugai, GSK, InflaRx, Novartis, Pfizer, Roche and Vifor Pharma, Grant/research support from: Ab2Bio, AbbVie, AstraZeneca, Bristol Myers Squibb, ChemoCentryx, GSK, InflaRx, Kiniksa, Nippon Kayaku, Novartis, Roche, and Sanofi (my institution received payments for participation in multicentre clinical trials and the institution or myself did not receive money for any other kind of research projects), Jane H. Bentley Shareholder of: GSK, Employee of: GSK, Jonathan Steinfeld Shareholder of: GSK, Employee of: GSK, Steven W Yancey Shareholder of: GSK, Employee of: GSK, Namhee Kwon Shareholder of: GSK, Employee of: GSK, Praveen Akuthota Paid instructor for: AstraZeneca, Consultant of: AstraZeneca, GSK, Sanofi, Grant/research support from: GSK, AstraZeneca and Regeneron, Paneez Khoury: None declared, Lee Baylis Shareholder of: GSK, Employee of: GSK, Michael Wechsler Consultant of: GSK, Genentech, Sanofi, Regeneron, AstraZeneca, Teva, Novartis, Boehringer Ingelheim, Sentien, and Equillium, Grant/research support from: National Institute of Allergy and Infectious Diseases and the National Heart, Lung, and Blood Institute