

Background: Ixekizumab (IXE) has demonstrated efficacy at week (wk) 16 which was maintained through 2 years (yrs) and was associated with a consistent safety profile in patients (pts) with r- and nr-axSpA, who are bDMARD-naïve and TNFi-experienced. 1-3
Objectives: To report safety and efficacy from the COAST programme at 3 yrs: 1 yr of the originating studies (COAST-V/W/X) and 2 yrs of COAST-Y.
Methods: COAST-Y (NCT03129100) is the phase 3, long-term extension study of the 3 originating studies COAST-V/W/X. Pts continued with the dose received at the end of the originating trial at week (wk) 52: either with 80 mg IXE every 4 wks (Q4W) or every 2 wks (Q2W). Pts assigned to adalimumab (ADA) or placebo (PBO) were re-randomised to IXE Q4W or Q2W at wk 16 in COAST-V and -W. Pts who received PBO for 52 wks in COAST-X were switched to IXE Q4W to continue in COAST-Y. Starting at wk 116 (wk 64 of COAST-Y), pts receiving IXE Q4W could have their dose escalated to Q2W. This analysis focused on pts receiving ≥1 dose of IXE Q4W, observed data while on IXE Q2W dose escalation are excluded. Continuous data are summarised as observed. Safety data while on IXE were analysed for pts who received ≥1 dose of IXE; observed data while on PBO or ADA are excluded.
Results: A total of 932 pts received ≥1 dose of IXE, 414 received ≥1 dose of IXE Q4W, and 562/932 (60%) pts completed 3 yrs of follow-up (PBO→IXE Q4W, 63/119 (53%); ADA→IXE Q4W, 29/44 (66%); and IXE Q4W→IXE Q4W, 114/251(45%)). Through 3 yrs, the most frequently reported treatment-emergent adverse events were infections [incidence rate (IR) 25.7/100 patient years (PY)] and injection site reactions [IR 7.4/100 PY]; the majority of which were mild/moderate in severity. Serious adverse events were reported at an IR of 4.8/100 PY, of which osteoarthritis was the most frequent at 0.4/100 PY. A total of 3 deaths were reported among all pts who received ≥1 dose of IXE [IR 0.1/100 PY]. For all pts, baseline disease activity (Ankylosing Spondylitis Disease Activity Score; ASDAS) was high (see
Baseline demographics and disease activity characteristics through 3 yrs. Data presented as mean (SD) unless otherwise specified.
| COAST-V | COAST-W | COAST-X | |||||
|---|---|---|---|---|---|---|---|
| PBO (N=87) | ADA (N=90) | IXE Q4W (N=81) | PBO (N=104) | IXE Q4W (N=114) | PBO (N=105) | IXE Q4W (N=96) | |
| Age | 43 (12) | 42 (11) | 41 (12) | 47 (13) | 47 (13) | 40 (12) | 41 (15) |
| Male, n (%) | 71 (83) | 73 (81) | 68 (84) | 87 (84) | 91 (80) | 44 (42) | 50 (52) |
| Symptom duration (years) | 16.6 (10.1) | 15.6 (9.3) | 15.8 (11.2) | 19.9 (11.6) | 18.8 (11.6) | 10.1 (8.3) | 11.3 (10.7) |
| HLA-B27, n (%) | 76 (89) | 82 (91) | 75 (93) | 86 (83) | 91 (80) | 77 (74) | 71 (75) |
| ASDAS | 3.9 (0.7) | 3.7 (0.8) | 3.7 (0.7) | 4.1 (0.8) | 4.2 (0.9) | 3.8 (0.9) | 3.8 (0.8) |
| BASDAI | 6.8 (1.2) | 6.7 (1.5) | 6.8 (1.3) | 7.3 (1.3) | 7.5 (1.3) | 7.2 (1.5) | 7.0 (1.5) |
| 3 years (observed ) | |||||||
| PBO→ | ADA→ | IXE | PBO→ | IXE | IXE | ||
| IXE Q4W | IXE Q4W | Q4W→ | IXE Q4W | Q4W→ | PBO→ | Q4W→ | |
| N=42 | N=44 | IXE Q4W | N=46 | IXE Q4W | IXE Q4W | IXE Q4W | |
| N=81 | N=114 | N=31 | N=56 | ||||
| ASDAS CFB | -1.9 (0.9) | -1.5 (0.9) | -1.9 (0.9) | -1.6 (1.0) | -1.7 (1.0) | 1.8 (1.0) | -1.7 (1.4) |
| ASDAS LDA, n (%) | 13/24 (54) | 21/29 (72) | 33/44 (75) | 7/20 (35) | 16/41 (39) | 13/19 (68) | 19/29 (66) |
| BASDAI CFB | -3.9 (1.9) | -3.5 (2.3) | -4.0 (2.2) | -3.7 (1.7) | -3.4 (2.2) | -4.4 (2.1) | -3.4 (2.7) |
| BASDAI50, n (%) | 15/24 (63) | 18/29 (62) | 31/44 (71) | 9/20 (45) | 20/41 (49) | 12/19 (63) | 16/29 (55) |
| ASAS40, n (%) | 13/24 (54) | 18/29 (62) | 30/44 (68) | 10/20 (50) | 23/41 (56) | 15/19 (79) | 17/29 (59) |
Conclusion: This analysis of a subset of pts in COAST-Y demonstrated that the safety profile is consistent with the established safety profile, with no new safety signals observed. IXE Q4W was efficacious (observed data) in all patients studied who remained on the treatment through 3 yrs.
REFERENCES:
[1]Dougados et al. Ann Rheum Dis 2020;79.
[2]Deodhar et al. Lancet 2020; 395.
[3]Braun et al. Ann Rheum Dis, 2021; 80: supp 1
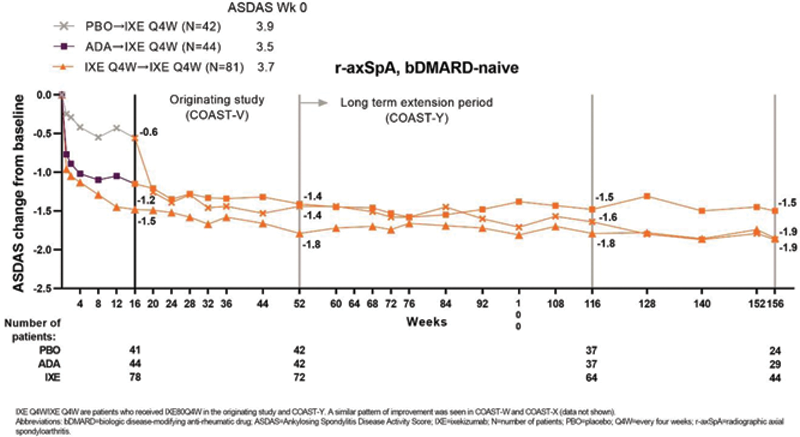
Observed mean CFB in ASDAS for pts treated with IXE Q4W in COAST-V. At wk 16, PBO pts received IXE Q4W.
Acknowledgements: The authors thank So Young Park, PhD, of Eli Lilly and Company for statistical review, and Edel Hughes, PhD, of Eli Lilly and Company for writing and process support.
Disclosure of Interests: Atul Deodhar Speakers bureau: AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Janssen, Novartis, Pfizer, UCB, Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Galapagos, Glaxo Smith & Kline, Janssen, Novartis, Pfizer, UCB, Grant/research support from: AbbVie, Eli Lilly and Company, Glaxo Smith & Kline, Novartis, Pfizer, UCB, Denis Poddubnyy Speakers bureau: AbbVie, Bristol-Myers Squibb, Eli Lilly and Company, MSD, Novartis, Pfizer, and UCB, Consultant of: AbbVie, Biocad, Eli Lilly and Company, Gilead, GlaxoSmithKline, Janssen, MSD, Novartis, Pfizer, Samsung Bioepis, and UCB, Grant/research support from: AbbVie, Eli Lilly and Company, MSD, Novartis, and Pfizer, Proton Rahman Speakers bureau: Abbott, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly and Company, Janssen, Novartis, and Pfizer., Grant/research support from: Janssen, Novartis, Rebecca Bolce Shareholder of: Eli Lilly and Company, Employee of: Eli Lilly and Company, Soyi Liu Leage Shareholder of: Eli Lilly and Company, Employee of: Eli Lilly and Company, Andris Kronbergs Shareholder of: Eli Lilly and Company, Employee of: Eli Lilly and Company, Caroline Johnson Shareholder of: Eli Lilly and Company, Employee of: Eli Lilly and Company, Ann Leung Employee of: Employee of Syneos Health, and a contractor for Eli Lilly and Company, Désirée van der Heijde Consultant of: AbbVie, Bayer, BMS, Cyxone, Eisai, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Eli Lilly and Company, Novartis, Pfizer, UCB Pharma, and Director of Imaging Rheumatology BV.