

Background: Certolizumab pegol (CZP) has demonstrated clinical efficacy in patients with active non-radiographic axial spondyloarthritis (nr-axSpA) and objective signs of inflammation during the 52-week (wk) placebo (PBO)-controlled period and 104 wk open-label (OL) safety follow-up extension (SFE) of the C-axSpAnd study. 1 There is, however, a paucity of data on the long-term efficacy of biologics in nr-axSpA according to patients’ baseline MRI and C-reactive protein (CRP) status.
Objectives: This post hoc analysis from C-axSpAnd aimed to evaluate whether patients’ baseline MRI and CRP status impacted long-term (3-year) clinical responses to CZP.
Methods: C-axSpAnd (NCT02552212) was a 3-year, phase 3, multicentre study. Adults (N=317) with nr-axSpA fulfilling the Assessment of SpondyloArthritis international Society (ASAS) classification criteria and objective signs of inflammation (CRP ≥ upper limit of normal (10 mg/L) [CRP+] and/or evidence of sacroiliitis on MRI [MRI+]) 2 were randomised 1:1 to PBO or CZP (400 mg at Wks 0, 2 and 4, then 200 mg every 2 wks [Q2W]) for 52 wks. 3 Those enrolled into the SFE received OL CZP (200 mg Q2W) for an additional 104 wks.
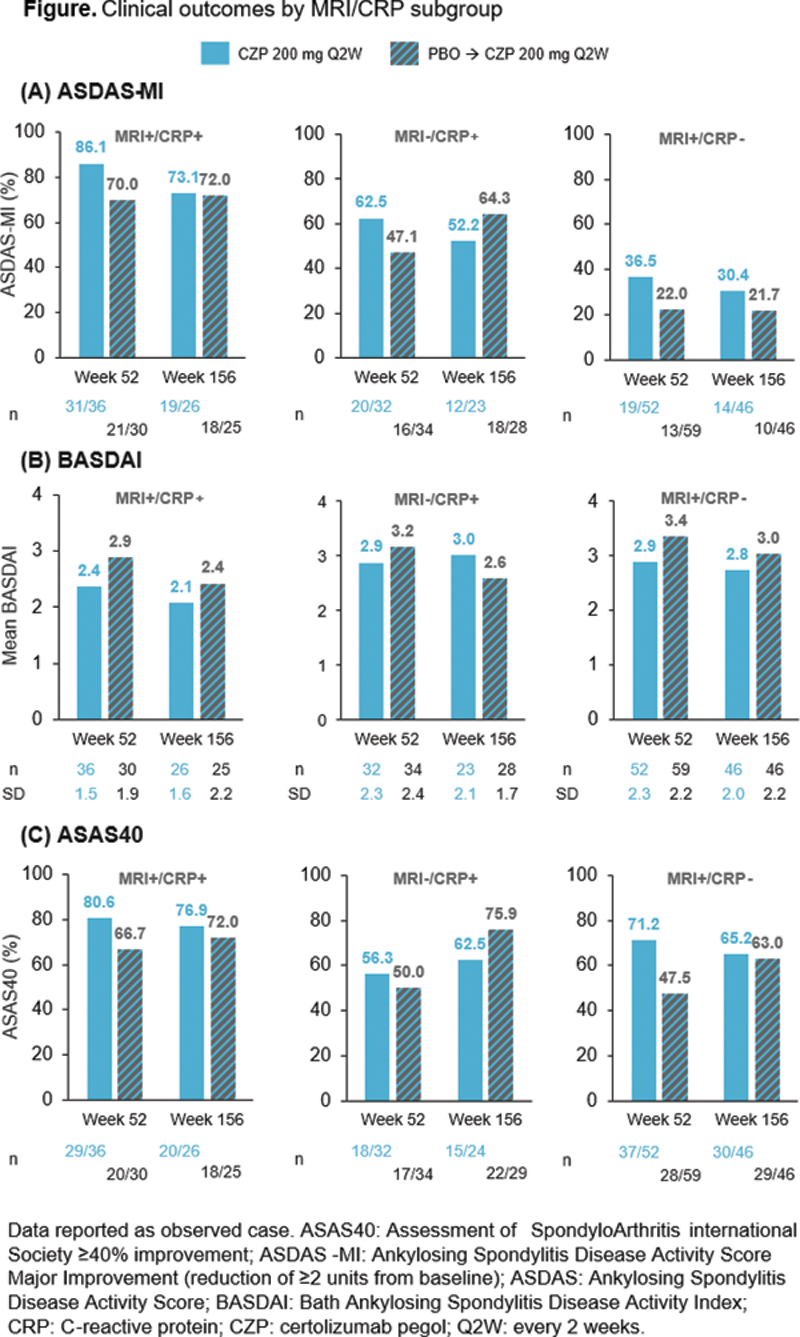
Ankylosing Spondylitis Disease Activity Score (ASDAS) and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) alongside the percentage of patients achieving ASDAS major improvement (ASDAS-MI, C-axSpAnd primary outcome) and ASAS 40% response (ASAS40) at Wks 52 and 156 were assessed according to prespecified subgroups based on MRI/CRP status (MRI+/CRP+, MRI−/CRP+, MRI+/CRP−). All data are reported as observed case.
Results: 243/317 (76.7%) patients entered the SFE, 120 from the group initially randomised to CZP (36 MRI+/CRP+, 32 MRI−/CRP+ and 52 MRI+/CRP−) and 123 from the initial PBO group (30 MRI+/CRP+, 34 MRI−/CRP+ and 59 MRI+/CRP−; 75/123 had switched to OL treatment in the 52 wk double-blind phase). 206/243 completed the SFE; 102/120 (85.0%) from the group initially randomised to CZP, 104/123 (84.6%) from the initial PBO group.
Among CZP-randomised patients, mean ASDAS was similar between timepoints (MRI+/CRP+: 1.6 at Wk 52 vs 1.6 at Wk 156; MRI−/CRP+: 2.1 vs 2.2; MRI+/CRP−: 1.7 vs 1.6), the percentage achieving ASDAS-MI was lower at Wk 156 compared to Wk 52 across all subgroups (
Similar results were shown for BASDAI, with mean scores for CZP-randomised patients sustained from Wk 52 to Wk 156 across all subgroups (
In CZP-randomised patients, ASAS40 responses were sustained at Wk 156 compared to Wk 52. An increased percentage of patients achieved ASAS40 in all MRI/CRP subgroups initially randomised to PBO at Wk 156 compared to Wk 52 (
Conclusion: In this analysis of patients with nr-axSpA and objective signs of inflammation, long-term clinical outcomes achieved after 1 year were generally sustained at 3 years across MRI+/CRP+, MRI−/CRP+ and MRI+/CRP− subgroups; ASDAS-MI was numerically highest in the MRI+/CRP+ subgroup.
REFERENCES:
[1]van der Heijde D. Arthritis Rheumatol 2021;73 (suppl 10);
[2]Lambert RG. Ann Rheum Dis 2016;75(11):1958–63;
[3]Deodhar A. Arthritis Rheumatol 2019;71(7):1101–11.
Acknowledgements: This study was funded by UCB Pharma. Editorial services were provided by Costello Medical and funded by UCB Pharma.
Disclosure of Interests: Philip Robinson Consultant of: Personal fees from AbbVie, Atom Biosciences, Eli Lilly, Gilead, Janssen, Novartis, Roche, Pfizer and UCB Pharma, Grant/research support from: Grant funding from Janssen, Novartis and UCB Pharma; meeting attendance support from Bristol Myers Squibb, Lilly, Pfizer and Roche, Walter P Maksymowych Consultant of: Honoraria/consulting fees from AbbVie, Boehringer Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer and UCB Pharma, Grant/research support from: Research grants from AbbVie and Pfizer; educational grants from AbbVie, Janssen, Novartis and Pfizer; Chief Medical Officer for CARE Arthritis Limited., Lianne S. Gensler Speakers bureau: Speaker for AbbVie, Eli Lilly, Novartis and UCB Pharma, Consultant of: Consulting fees from AbbVie, Celgene, Eli Lilly, Janssen, Novartis and UCB Pharma, Martin Rudwaleit Speakers bureau: Speaker for AbbVie, Eli Lilly, Novartis and UCB Pharma, Consultant of: Consulting fees from AbbVie, Celgene, Eli Lilly, Janssen, Novartis and UCB Pharma, Bengt Hoepken Shareholder of: Stockholder of UCB Pharma, Employee of: Employee of UCB Pharma, Lars Bauer Shareholder of: Stockholder of UCB Pharma, Employee of: Employee of UCB Pharma, Thomas Kumke Shareholder of: Stockholder of UCB Pharma, Employee of: Employee of UCB Pharma, Mindy Kim Shareholder of: Stockholder of UCB Pharma, Employee of: Employee of UCB Pharma, Atul Deodhar Speakers bureau: Speaker for Janssen, Novartis and Pfizer, Consultant of: Consulting fees from AbbVie, Amgen, Aurinia, Bristol Myers Squibb, Celgene, Eli Lilly, GSK, Janssen, MoonLake, Novartis, Pfizer and UCB Pharma, Grant/research support from: Research grants from AbbVie, Eli Lilly, GSK, Novartis, Pfizer and UCB Pharma