

Background: For patients with psoriatic arthritis (PsA), delays in diagnosis and treatment can lead to permanent radiographic damage, even early in the course of disease. 1 In the phase 3 FUTURE 5 study (NCT02404350), treatment with secukinumab (SEC) was shown to inhibit progression of structural damage through Week 104 in patients with PsA. 2 However, the effect of disease duration on inhibition of radiographic progression by SEC has not been characterized.
Objectives: This post hoc analysis explored relationships between time since diagnosis (TSD) of ≤1 year vs >1 year and radiographic progression among patients with PsA receiving SEC over 2 years in FUTURE 5.
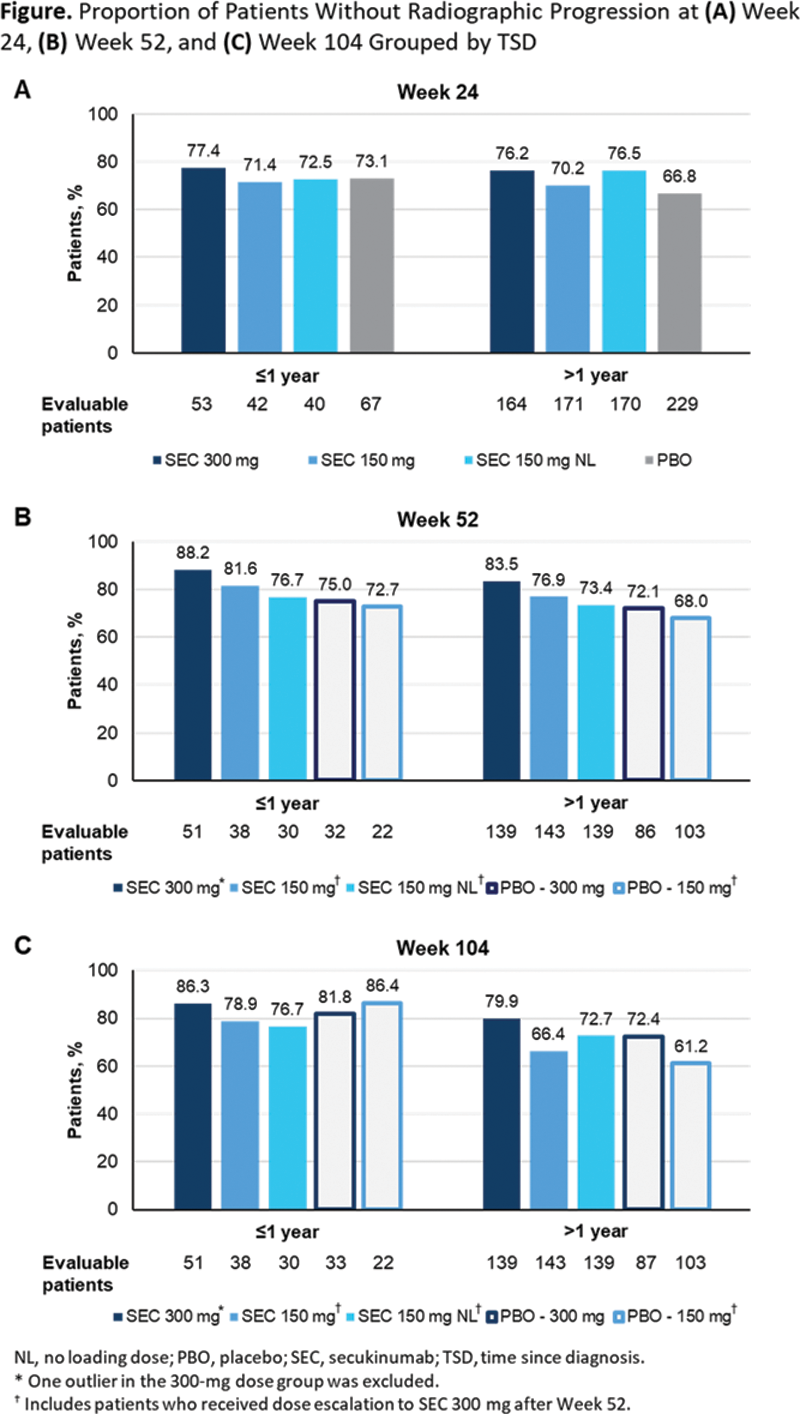
Methods: Patient data from FUTURE 5 were stratified by TSD ≤1 year vs >1 year and analyzed by treatment arm. Through Week 24, patients received SEC 300 or 150 mg with subcutaneous loading dose (LD), SEC 150 mg without LD, or placebo (PBO) (period 1). After Week 24, patients receiving PBO were switched to SEC 300 or 150 mg (period 2), and a protocol amendment allowed those with suboptimal clinical response to SEC 150 mg to escalate to SEC 300 mg after Week 52 per investigator judgment. 2 The proportion of patients with no radiographic progression, defined as change from baseline in van der Heijde total modified Sharp score ≤0.0, was analyzed at Weeks 24, 52, and 104. Mean total Sharp score was evaluated at baseline, and mean change from baseline was determined at Weeks 24, 52, and 104.
Results: Of 996 patients with PsA included here, 217 (21.8%) had a TSD ≤1 year and 779 (78.2%) had a TSD >1 year. At baseline, patients with TSD >1 year had greater radiographic damage than patients with TSD ≤1 year as determined by mean total Sharp score (
Baseline Total Sharp Score and Change From Baseline at Weeks 24, 52, and 104 by TSD
| Total Sharp score | TSD ≤1 year | TSD >1 year | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Period 1 | SEC 300 mg n = 54 | SEC 150 mg n = 46 | SEC 150 mg NL n = 43 | PBO n = 74 | SEC 300 mg n = 168 | SEC 150 mg n = 174 | SEC 150 mg NL n = 179 | PBO n = 258 | ||
| Baseline, mean (SD) | 8.02 (20.77) | 8.82 (12.06) | 12.74 (33.67) | 8.84 (20.42) | 14.37 (24.17) | 14.67 (28.01) | 15.56 (37.52) | 17.34 (41.21) | ||
| Week 24 change from baseline, mean (SD) | 0.05 (0.72) | −0.08 (1.40) | −0.61 (5.25) | 0.76 (2.05) | 0.09 (1.37) | 0.23 (1.24) | 0.03 (2.05) | 0.42 (1.56) | ||
| Period 2 | SEC 300 mg* n = 54 | SEC 150 mg † n = 46 | SEC 150 mg NL † n = 43 | PBO ‒ 300 mg n = 40 | PBO ‒ 150 mg † n = 30 | SEC 300 mg* n = 168 | SEC 150 mg † n = 174 | SEC 150 mg NL † n = 179 | PBO ‒ 300 mg n = 113 | PBO ‒ 150 mg † n = 123 |
| Week 52 change from baseline, mean (SD) | 0.05 (0.48) | −0.03 (1.22) | 0.35 (2.25) | 0.22 (0.70) | 0.18 (0.75) | −0.07 (1.16) | 0.26 (1.96) | 0.26 (1.05) | 0.16 (0.94) | 0.40 (2.00) |
| Week 104 change from baseline, mean (SD) | 0.06 (0.63) | 0.11 (0.99) | 0.20 (2.71) | 0.11 (0.68) | −0.07 (0.50) | 0.11 (2.00) | 0.62 (2.94) | 0.46 (2.08) | 0.12 (0.90) | 0.81 (2.66) |
NL, no loading dose; PBO, placebo; SEC, secukinumab; TSD, time since diagnosis.
* One outlier in the 300-mg dose group was excluded.
† Includes patients who received dose escalation to SEC 300 mg after Week 52.
Conclusion: SEC resulted in low rates of radiographic progression through 2 years of treatment among patients in FUTURE 5, regardless of time since PsA diagnosis.
REFERENCES:
[1]Haroon M, et al. Ann Rheum Dis . 2015;74:1045-50.
[2]Mease P, et al. RMD Open. 2021;7:e001600.
Acknowledgements: This study was funded by Novartis Pharmaceuticals Corporation. Medical writing support was provided by Richard Karpowicz, PhD, CMPP, of Health Interactions, Inc, and was funded by Novartis Pharmaceuticals Corporation. This abstract was developed in accordance with Good Publication Practice (GPP3) guidelines. Authors had full control of the content and made the final decision on all aspects of this publication.
Disclosure of Interests: Christopher T. Ritchlin Consultant of: AbbVie, Amgen, Eli Lilly, Janssen, Pfizer, Novartis, Gilead, and UCB, Ana-Maria Orbai Consultant of: Bristol Myers Squibb, Janssen, Lilly, Novartis, Pfizer, and UCB, Grant/research support from: to Johns Hopkins University from AbbVie, Amgen, Celgene, Horizon, Janssen, Lilly, and Novartis, Bhumik Parikh Employee of: Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA, Corine Gaillez Employee of: Novartis Pharma AG, Basel, Switzerland, Xiangyi Meng Employee of: Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA, Philip J Mease Speakers bureau: AbbVie, Amgen, Janssen, Eli Lilly, Novartis, Pfizer, and UCB, Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Galapagos, Gilead, GlaxoSmithKline, Janssen, Eli Lilly, Novartis, Pfizer, Sun Pharma, and UCB, Grant/research support from: AbbVie, Amgen, Bristol Myers Squibb, Celgene, Gilead, Janssen, Eli Lilly, Novartis, Pfizer, Sun Pharma, and UCB