

Background: Apremilast, an inhibitor of the phosphodiesterase 4, is indicated for Psoriatic Arthritis (PsA) treatment. The 3 years retention rate, an outcome indirectly related to efficacy, observed in clinical trials [1] is 55,5%. A single subsequent real world setting study [2] suggested a lower efficacy as it reported that the six months retention rate was about 57%.
Objectives: The main aim of this retrospective observational study is the assessment of apremilast 3 years retention rate in a real world PsA patients’ cohort. Moreover, the secondary objective is reporting the reasons of apremilast suspension and the most relevant factor related to treatment persistence.
Methods: In thirteen Italian rheumatological referral centers, all PsA consecutive patients who received apremilast were enrolled. Anamnestic data, treatment history and PsA disease activity (DAPSA) at baseline and after 6 and 12 months were recorded. The Kaplan-Meier curve and the Cox analysis computed the apremilast retention rate and treatment persistence-related risk factors. A p-value < 0.05 was considered statistically significant.
Results: The three-hundred-twenty-four enrolled patients (median age 60 [InterQuartile Range IQR 52-67] yrs; female prevalence 57,0%) median observation period was 17 [IQR 7-36] months (6848 patients-months). The apremilast retention rate at 6, 12 and 36 months was, respectively, 95%, 86% and 66% (
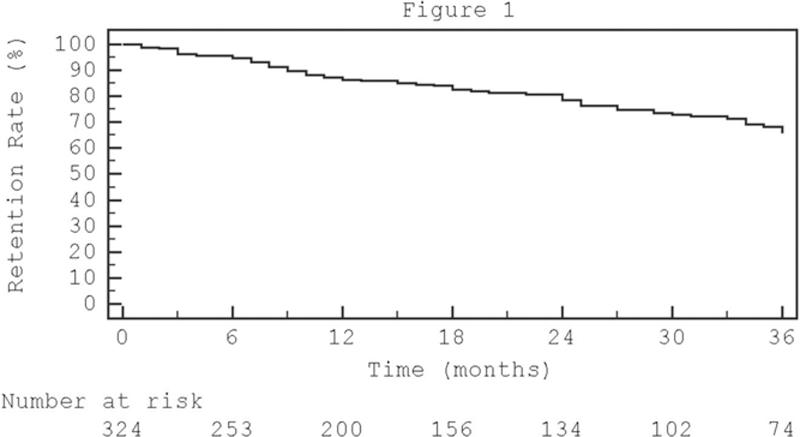
Conclusion: Almost two third of PsA patients receiving apremilast were still in treatment after 3 years. The study’s data, confirmed its efficacy and safety profile. Apremilast appear a good treatment choice in patients with oligo articular PsA or burdened by severe comorbidities.
REFERENCES:
[1]Mease et al. ACR Open Rheumatology (2020)
[2]Favalli et al Clin Exp Rheum (2020)
Disclosure of Interests: Alarico Ariani Speakers bureau: Zentiva, Consultant of: Boeringher, Amgen, Bristol-Meyers-Squibb, Novartis, Sanofi, Novo Nordisk, Lilly, Janssen, Bruno Farmaceutici, Simone Parisi: None declared, Patrizia Del Medico: None declared, antonella farina: None declared, elisa visalli: None declared, Aldo Molica Colella: None declared, Federica Lumetti Consultant of: Amgen, rosalba caccavale: None declared, Palma Scolieri: None declared, Romina Andracco: None declared, Francesco Girelli: None declared, Elena Bravi: None declared, Matteo Colina: None declared, Veronica Franchina: None declared, Ilaria Platé: None declared, eleonora Di Donato Consultant of: Novartis, Giorgio Amato: None declared, Carlo Salvarani: None declared, Francesco De Lucia: None declared, Daniele Santilli Consultant of: Novartis, eugenio arrigoni: None declared, Flavio Mozzani Consultant of: Novartis, Abbvie, Rosario Foti: None declared, Gilda Sandri: None declared, Vincenzo Bruzzese: None declared, Marino Paroli: None declared, Enrico Fusaro: None declared, Andrea Becciolini: None declared