

Background: Guselkumab (GUS), an IL-23p19-subunit inhibitor, demonstrated efficacy and a favorable safety profile in patients (pts) with psoriasis (PsO) and psoriatic arthritis (PsA). In the Phase 3, double-blind, placebo (PBO)-controlled DISCOVER-2 study, GUS 100 mg every 4 or 8 weeks (Q4W or Q8W) significantly improved joint and skin symptoms; GUS-treated pts had smaller mean changes in radiographic progression vs. PBO at W24. 1 Low rates of radiographic progression were observed through 2 years among GUS-treated pts, regardless of dosing regimen. 2,3
Objectives: Determine whether earlier clinical improvement predicts long-term radiographic progression through 2 years in DISCOVER-2.
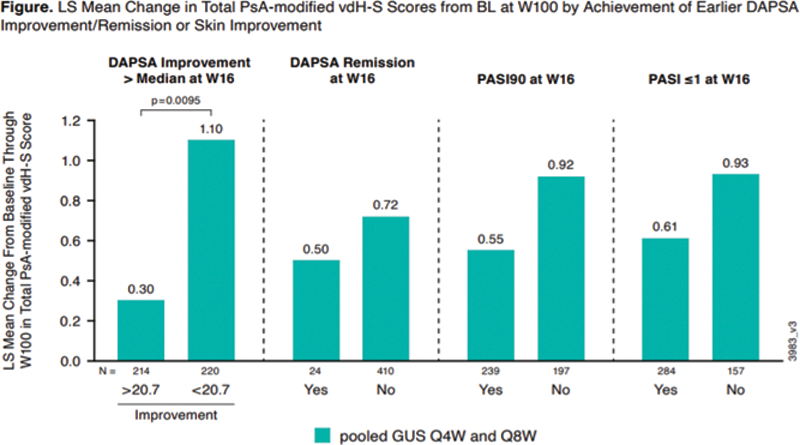
Methods: DISCOVER-2 included biologic-naïve pts with active PsA (≥5 swollen and ≥5 tender joint counts [SJC/TJC]; CRP ≥0.6 mg/dL) randomized (1:1:1) to GUS 100 mg Q4W; GUS 100 mg at W0, W4, then Q8W; or PBO with crossover to GUS 100 mg Q4W (PBO→Q4W) at W24. For pts randomized to GUS Q4W or Q8W, predictive models (mixed linear) were developed post-hoc to assess the associations of earlier (at W16) improvement in disease activity (DAPSA remission, DAPSA Improvement, DAPSA Improvement more than the median of 20.7 [>20.7]) or skin improvement (PASI90, PASI≤1) with changes in total PsA modified van der Heijde-Sharp [vdH-S] score through W100, after adjusting for known baseline (BL) determinants of radiographic progression (vdH-S score, age, gender, and CRP).
Results: PsA duration, CRP, and SJC at BL weakly correlated with BL vdH-S score. No correlation was seen between BL PASI and BL vdH-S score (
Correlation of Select BL Disease Characteristics with BL vdH-S Score Among GUS Randomized Pts
| BL Determinants | Spearman’s correlation coefficient | p-value |
|---|---|---|
| Age | 0.27335 | <.0001 |
| CRP | 0.28181 | <.0001 |
| PASI Score | 0.03078 | 0.5153 |
| PsA Duration | 0.37070 | <.0001 |
| PsO Duration | 0.20509 | <.0001 |
| SJC (66) | 0.26321 | <.0001 |
Conclusion: In GUS-treated biologic-naïve pts with active PsA, following adjustment for known BL determinants of radiographic progression, earlier (W16) DAPSA improvement was a significant predictor of less radiographic progression through W100; DAPSA remission and skin improvement at W16 each showed a numerical trend toward less radiographic progression through W100.
REFERENCES:
[1]Mease PJ, et al. Lancet . 2020;395:1126–36.
[2]McInnes IB, et al. Arthritis Rheumatol . 2021;73:604-16.
[3]McInnes IB, et al. Arthritis Rheumatol. 2021 Nov 1. doi: 10.1002/art.42010. Online ahead of print.
Disclosure of Interests: Philip J Mease Speakers bureau: AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, Sun Pharma, and UCB, Consultant of: AbbVie, Aclaris, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Inmagene, Janssen, Novartis, Pfizer, Sun Pharma, and UCB, Grant/research support from: AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, Janssen, Novartis, Pfizer, Sun Pharma, and UCB, Alice B Gottlieb Speakers bureau: AnaptsysBio, Avotres Therapeutics, Beiersdorf, Boehringer Ingelheim, Bristol-Myers Squibb, Incyte, GSK, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sun Pharmaceutical Industries, Inc., UCB, and Dermavant, Consultant of: AnaptsysBio, Avotres Therapeutics, Beiersdorf, Boehringer Ingelheim, Bristol-Myers Squibb, Incyte, GSK, Janssen, LEO Pharma, Eli Lilly, Novartis, Pfizer, Sun Pharmaceutical Industries, Inc., UCB, and Dermavant, Grant/research support from: Boehringer Ingelheim, Incyte, Janssen, Novartis, UCB, Xbiotech, and Sun Pharma, Alexis Ogdie Consultant of: Abbvie, Amgen, BMS, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB, Grant/research support from: University of Pennsylvania from Abbvie, Pfizer and Novartis and to Forward from Amgen, Iain McInnes Shareholder of: Causeway Therapeutics, and Evelo Compugen, Consultant of: Astra Zeneca, AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Cabaletta, Compugen, GSK, Gilead, Janssen, Novartis, Pfizer, Sanofi, Roche, and UCB, Grant/research support from: Astra Zeneca, Bristol-Myers Squibb, Amgen, Eli Lilly, GSK, Janssen, Novartis, Roche, and UCB, Soumya D Chakravarty Shareholder of: Johnson & Johnson, Employee of: Janssen Scientific Affairs, LLC and Drexel University College of Medicine, Emmanouil Rampakakis Consultant of: Janssen, Employee of: JSS Medical Research, Alexa Kollmeier Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Xie L Xu Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, May Shawi Shareholder of: Johnson & Johnson, Employee of: Janssen Pharmaceutical Companies of Johnson & Johnson, Frederic Lavie Shareholder of: Johnson & Johnson, Employee of: Janssen Cilag Global Medial Affairs, Mitsumasa Kishimoto Speakers bureau: AbbVie, Amgen-Astellas BioPharma, Asahi-Kasei Pharma, Astellas, Ayumi Pharma, BMS, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Kyowa Kirin, Novartis, Pfizer, Tanabe-Mitsubishi, Teijin Pharma, and UCB, Consultant of: AbbVie, Amgen-Astellas BioPharma, Asahi-Kasei Pharma, Astellas, Ayumi Pharma, BMS, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Kyowa Kirin, Novartis, Pfizer, Tanabe-Mitsubishi, Teijin Pharma, and UCB, Proton Rahman Speakers bureau: Janssen, Consultant of: AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer, and UCB, Grant/research support from: Janssen and Novartis