

Background: Controlling or improving musculoskeletal disease activity of psoriatic arthritis (PsA) (eg, enthesitis and associated pain) is a treatment priority for patients, rheumatologists, and dermatologists. 1 Enthesitis is the cardinal lesion in PsA and is immunogenetically and experimentally linked to the interleukin-23 (IL-23) pathway. 2 Risankizumab (RZB), a humanized immunoglobulin G1 monoclonal antibody that specifically inhibits IL-23 by binding to its p19 subunit, was studied in a phase 3 adult PsA program (KEEPsAKE clinical trials). 3,4 Pooled analyses from the program demonstrated the efficacy of RZB to treat enthesitis and pain associated with PsA, and increase the proportion of patients whose enthesitis resolved compared with placebo (PBO) in those patients who had an inadequate response or intolerance to ≥1 conventional synthetic disease-modifying antirheumatic drugs (KEEPsAKE 1 and 2) and/or ≤ 2 biological therapies (KEEPsAKE 2).
Objectives: To investigate whether patients without enthesitis at baseline (BL) (Leeds Enthesitis Index [LEI] = 0 at BL) remained enthesitis-free through week (W) 52, patients with enthesitis at BL (LEI > 0 at BL) had resolution of enthesitis through W52, and if greater pain relief was achieved with RZB 150 mg in patients with enthesitis at BL vs PBO up to W24.
Methods: The study design and primary results of KEEPsAKE 1 (NCT03675308) and KEEPsAKE 2 (NCT03671148) have been previously reported. 3,4 Briefly, patients were randomized to receive RZB 150 mg or PBO subcutaneously at weeks 0, 4, and 16 during a 24-week, double-blind treatment period; at W28 all patients received open label RZB 150 mg. For this post hoc analysis, the RZB 150 mg and PBO groups were pooled across the 2 studies. Pain reductions (as measured by change from BL in visual analogue scale [VAS] scores) were assessed at each time point through W24 among patients with enthesitis at BL (LEI > 0 at BL) using mixed-effect model repeated measurement analysis. Additional enthesitis analyses were calculated on the data as observed.
Results: Across the pooled population, over 60% of patients in each treatment group had enthesitis at BL (RZB=444/707 [63%]; PBO=448/700 [64%]). Conversely, 37% (263/707) and 36% (252/700) had no enthesitis (LEI=0) at BL among those randomized to RZB and PBO, respectively. Among enthesitis-free patients at BL (LEI=0 at BL), 84.7% on PBO and 90% on RZB remained free of enthesitis through W24; by W52, approximately 93% of patients in both groups (RZB and PBO to RZB) remained enthesitis free. A numerically higher proportion of patients with enthesitis at BL (LEI > 0 at BL) treated with RZB (52.1%) achieved an enthesitis-free state at W24 vs PBO (41.8%); similar proportions achieved an enthesitis-free state at W36 and W52 during open label treatment (
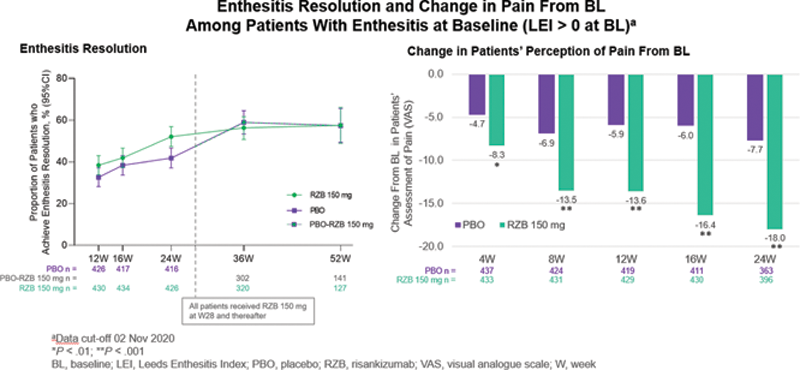
Conclusion: Long-term maintenance of an enthesitis-free state (LEI = 0) was similar between the RZB 150 mg and PBO groups, with approximately 93% of patients remaining free of enthesitis at W52. For LEI > 0 patients, the RZB 150-mg group had numerically more patients whose enthesitis resolved at W24, and similar proportions were observed at W52 after the open label switch. Patients with enthesitis at BL treated with RZB 150 mg had statistically greater improvements in pain compared with patients taking PBO starting at W4 through to W24.
REFERENCES:
[1]Orbai A-M, et al. Ann Rheum Dis. 2017;76:673–680.
[2]Stavre Z, et al. Arthritis Res Ther . 2022;24(1):24.
[3]Kristensen LE, et al. Ann Rheum Dis. 2021;0:1–7.
[4]Östör A, et al. Ann Rheum Dis. 2021;0:1–8.
Acknowledgements: AbbVie Inc. participated in the study design; study research; collection, analysis, and interpretation of data; and writing, reviewing, and approving this abstract for submission. All authors had access to the data; participated in the development, review, and approval of the abstract; and agreed to submit this abstract to EULAR 2022 for consideration as a poster or oral presentation. No honoraria or payments were made for authorship. AbbVie and the authors thank all study investigators for their contributions and the patients who participated in this study. AbbVie funded the research for this study and provided writing support for this abstract. Medical writing assistance, funded by AbbVie, was provided by Kersten Reich, MPH, and Nancy Niguidula, DPH, of JB Ashtin.
Disclosure of Interests: Marina Magrey Consultant of: MM has received consulting fees from UCB, Novartis, Eli Lilly, Pfizer, and Janssen., Grant/research support from: MM received research grants from Amgen, AbbVie, and UCB Pharma, Manish Jain Consultant of: MJ received consulting fees from Amgen, Abbvie, Eli Lilly, Pfizer, and Novartis., Grant/research support from: MJ received research support from Amgen, Abbvie, Eli Lilly, Pfizer, and Novartis., R Ranza Speakers bureau: RR is a member of speaker bureaus for AbbVie, Janssen, Novartis, and Pfizer, Consultant of: RR is a consultant for AbbVie, Janssen, Novartis, and Pfizer, Jayne Stigler Shareholder of: JS may hold AbbVie stock or stock options., Employee of: JS is a full-time employee of AbbVie., Erin McDearmon-Blondell Shareholder of: EMB may hold AbbVie stock or stock options., Employee of: EMB is a full-time employee of AbbVie., Cuiyong Yue Shareholder of: CY may hold AbbVie stock or stock options., Employee of: CY is a full-time employee of AbbVie., Byron Padilla Shareholder of: BP may hold AbbVie stock or stock options., Employee of: BP is a full-time employee of AbbVie., Christian Kaufmann Shareholder of: CK may hold AbbVie stock or stock options., Employee of: CK is a full-time employee of AbbVie., Dennis McGonagle Speakers bureau: DM is a member of speaker bureaus for AbbVie, Janssen, Novartis, and Pfizer., Grant/research support from: DM received research grants from AbbVie, Janssen, Novartis, and Pfizer, UCB, BMS, Celgene.