

Background: Pain in patients (pts) with psoriatic arthritis (PsA) has multifaceted origins; sustained improvement is difficult to achieve. 1 Guselkumab (GUS), a fully human monoclonal antibody that selectively inhibits IL-23, is effective in treating multiple domains of PsA including joint, skin, and entheseal symptoms, and also elicits long-lasting improvements in pt-reported pain in the DISCOVER-1&2 trials of pts with active PsA. 2
Objectives: These post hoc analyses were conducted to identify determinants of changes in pt-reported pain in PsA pts using pooled data through 1 year of DISCOVER-1&2.
Methods: Enrolled adult pts had active PsA despite standard therapies. DISCOVER-1 pts had ≥3 swollen and ≥3 tender joints and C-reactive protein (CRP) ≥0.3 mg/dL; DISCOVER-2 pts had ≥5 swollen and ≥5 tender joints and CRP ≥0.6 mg/dL. 31% of DISCOVER-1 pts received 1-2 prior tumor necrosis factor inhibitors; DISCOVER-2 pts were biologic-naïve. Pts were randomized 1:1:1 to GUS 100 mg every 4 weeks (wks) (Q4W); GUS 100 mg at W0, W4, then every 8 wks (Q8W); or placebo (PBO); PBO pts crossed over to GUS 100 mg Q4W at W24. Determinants with a statistically important effect (p<0.15) on pain (0-100 mm Visual Analogue Scale) in univariate Repeated Measures Generalized Linear Mixed Effects Models were included in a multivariate model employing backward stepwise selection (P out =0.1) to identify independent determinants of pain improvement over 24 wks; the model was then tested separately in pts treated with PBO (through W24) and with GUS (through W24 and through W52).
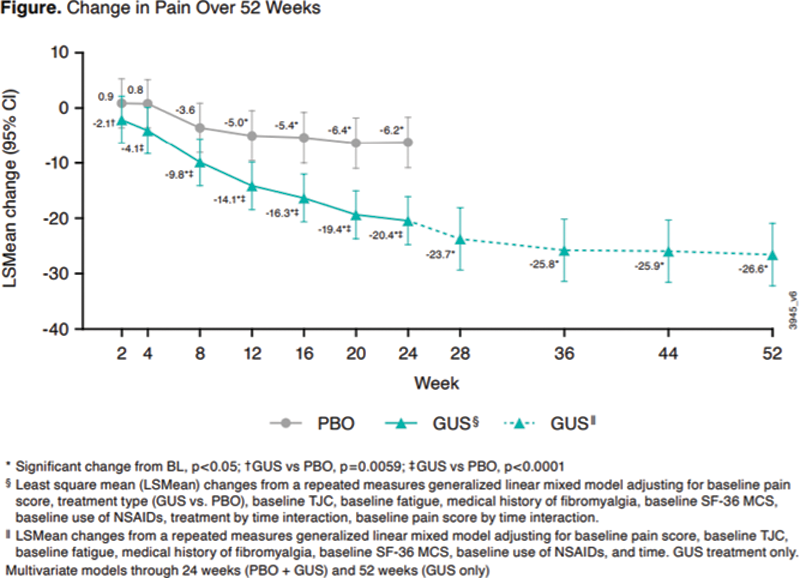
Results: GUS was associated with significantly greater improvement in pain compared to PBO as early as 2 wks post-treatment; there was a significant interaction between treatment group and time, with effect of GUS on pain continuously enhanced through W24. Higher baseline (BL) pain score, worse mental health (assessed with the Short-Form-36 Mental Component Summary [SF-36 MCS] score), and lower fatigue level and lower tender joint count [TJC] were also associated with significantly greater pain improvements at W24, while background use of NSAIDs was a negative predictor of pain improvement (
Significant Predictors of Change in Pain (W24 and W52 )
| BL Determinant | W24 | W52 |
|---|---|---|
| Estimate (95% CL) | Estimate (95% CL) | |
| Pain score | -0.62 (-0.69:-0.55) ‡ | -0.75 (-0.83:-0.67) ‡ |
| Fatigue | -0.38 (-0.50:-0.27) ‡ | -0.37 (-0.53:-0.22) ‡ |
| SF-36 MCS | 0.20 (0.11:0.30) ‡ | 0.11 (-0.02:0.24) |
| TJC | 0.13 (0.06:0.19) † | 0.12 (0.04:0.21) † |
| NSAID use (Y vs N) | 2.29 (0.62:3.96) † | 2.76 (0.55:4.98) * |
* p <0.05; † p <0.01; ‡ p ≤0.0001
(-25.9, -3.6) comparable to non-FM pts at W24, while pain improvement in pts with no FM was -22.2 (-24.0, -20.4).
Conclusion: Early significant effects of GUS on pain were enhanced through 1 year. Significant predictors of change in pain were consistent at W24 and W52, with the exception of mental health measures. The impact of mental status on pt-reported pain and the potential for GUS to improve pain in pts with FM warrant further consideration.
REFERENCES:
[1]Gudu T et al. Expert Rev Clin Immunol 2018;14(5):405-17.
[2]Nash P et al. ACR Convergence 2021;Nov 5-9 (Poster 21-1368).
Disclosure of Interests: Peter Nash Grant/research support from: Janssen, Abbvie, Pfizer, Novartis, Lilly, Gilead, Roche, Sandoz, Celgene, Sun, Boehringer, and Bristol Myers Squibb, Christopher T. Ritchlin Consultant of: UCB Pharma, Amgen, AbbVie, Lilly, Pfizer, Novartis, Gilead, Janssen, Grant/research support from: UCB Pharma, AbbVie, Amgen, Proton Rahman Consultant of: AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer, and UCB, Grant/research support from: Janssen and Novartis, May Shawi Shareholder of: Johnson & Johnson, Employee of: Janssen Pharmaceutical Companies of Johnson & Johnson, Emmanouil Rampakakis Consultant of: Janssen, Employee of: JSS Medical Research, YoungJa Lee Shareholder of: Johnson & Johnson, Employee of: Janssen Asia Pacific, Alexa Kollmeier Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Xie L Xu Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Jonathan Sherlock Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Daniel Cua Shareholder of: Johnson & Johnson, Employee of: Janssen Research & Development, LLC, Saakshi Khattri Speakers bureau: AbbVie, Eli Lilly, Glenmark, Ichnos Sciences, Janssen, Novartis, Pfizer, and UCB, Consultant of: AbbVie, Eli Lilly, Glenmark, Ichnos Sciences, Janssen, Novartis, Pfizer, and UCB, Enrique Soriano Speakers bureau: AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Janssen, Novartis, Pfizer, Roche, and UCB, Consultant of: AbbVie, Janssen, Novartis, and Roche, Grant/research support from: AbbVie, Janssen, Novartis, Pfizer, Roche, and UCB, Dennis McGonagle Grant/research support from: AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, and UCB