

Background: Changes in the integrity of the intestinal wall may be implicated in the gut-joint axis of inflammatory arthritis. 1 Yet, the gut barrier is only poorly evaluated in psoriatic arthritis (PsA). 2
Objectives: In this exploratory study, we evaluated intestinal permeability before and 26 weeks after one faecal microbiota transplantation (FMT) or sham intervention in adults with PsA.
Methods: We have previously reported the clinical results of a 26-week, double-blind, parallel-group, 1:1 randomised, sham-controlled, superiority trial of gastroscopic-guided FMT as an add-on treatment to methotrexate in 31 adults with active peripheral PsA (FLORA trial, NCT03058900). 3 The primary efficacy endpoint was the proportion of participants who experienced treatment failure through 26 weeks, defined as need for more than one intra-articular glucocorticoid injection and/or anti-TNFα inhibition. We encouraged patients not to take nonsteroidal anti-inflammatory drugs during the trial. The FMT material was obtained from one of four healthy blood donors. As part of the trial, we performed a lactulose and mannitol test (L:M test) at baseline (n=31) and at the final 26-week visit (n=26) to assess the permeability of the intestinal wall (higher L:M ratios indicate higher permeability). After an overnight fasting, patients provided a urine sample before ingesting 10 g of lactulose and 5 g of D-mannitol. Samples were collected after 3 hours and stored at −80°C until analysis. No food or drinking (except for water) was allowed during the test. We measured the lactulose-to-mannitol ratio in the urine samples using a Waters Acquity UPLC system coupled to a high-resolution mass spectrometer Waters Xevo G2 QToF (Waters Corp., Milford, MA, USA). MassLynx software (Waters Corporation) was used for data acquisition and visual inspection. We used StataSE-64 to perform the Wilcoxon rank sum and the matched-pairs signed-rank test. Data is presented as median and range. The level of significance was set to 0.05.
Results: At baseline, no significant difference was observed in the L:M ratio between donors (n=4) and patients (n=31) (0.0065 [0–0.063] vs 0.014 [0–0.28]; p=0.50). The L:M ratio increased from baseline to week 26 in both the FMT (0.0020 [-0.27 – 0.32] and the sham group (0.0046 [-0.012 – 0.088]), but only in the sham group differed the baseline L:M ratio significantly from the one measured at week 26 (p=0.92 [FMT] and p=0.032 [sham]). The patients who were classified as treatment failures during the trial (n=7) had a significantly higher L:M ratio at week 26 compared to the patients who were non-failures (n=19) (0.027 [0.017 – 0.33]) vs 0.012 [0 – 0.064], p=0.01), please see
L:M ratios at week 26 in treatment failures (n=7) and non-failures (n=19), respectively. Higher L:M ratios indicate higher intestinal permeability.
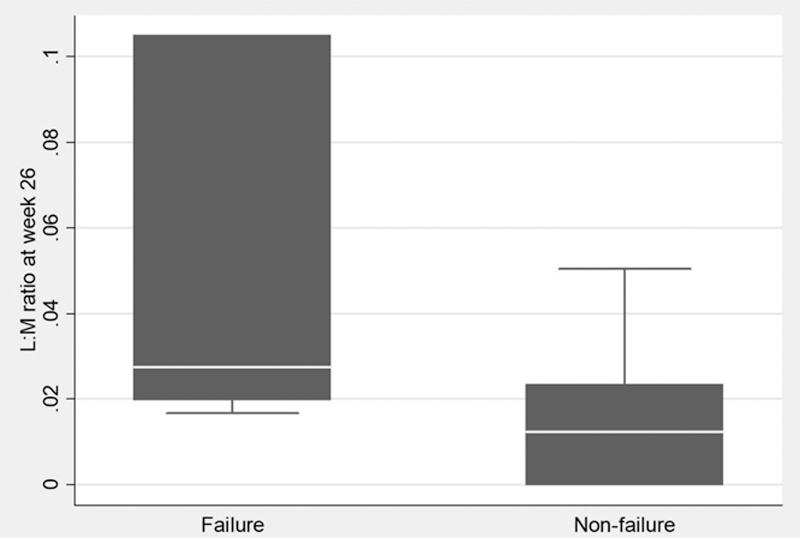
Conclusion: In the FLORA trial, intestinal permeability evaluated by the L:M test did not differ significantly between donors and patients at baseline. Whether the higher intestinal permeability observed in patients classified as treatment failures compared to non-failures at week 26 can be attributed to differences in disease activity and/or the instigation of additional immunosuppression in the failure group during the trial needs further investigation.
REFERENCES:
[1]Gracey E, Vereecke L, McGovern D, et al. Revisiting the gut-joint axis: links between gut inflammation and spondyloarthritis. Nat Rev Rheumatol . 2020;16(8):415-433.
[2]Hecquet S, Totoson P, Martin H, et al. Intestinal permeability in spondyloarthritis and rheumatoid arthritis: A systematic review of the literature. Semin Arthritis Rheum . 2021;51(4):712-718.
[3]Kragsnaes MS, Kjeldsen J, Horn HC, et al. Safety and efficacy of faecal microbiota transplantation for active peripheral psoriatic arthritis: an exploratory randomised placebo-controlled trial. Ann Rheum Dis . 2021;80(9):1158-1167.
Disclosure of Interests: None declared