

Background: Osteoporosis (OP) is a frequent complication identified in patients with rheumatoid arthritis (RA). Effective treatment must be provided to treat OP in RA (RAOP). Denosumab (DMB) is a promising drug, currently being used for the treatment of RAOP. Although DMB was determined to be effective in a long-term FREEDOM extension trial [1] for treating postmenopausal OP, its efficacy in the treatment of RAOP in real-world is not be fully evaluated.
Objectives: This retrospective study assessed the five-year treatment outcome of DMB in Japanese patients with RAOP.
Methods: Data from the Toyohashi RA Database (TRAD) was used, which is a collection of single-center retrospective data. Our study included 65 female patients with RAOP for whom DMB treatment was initiated between October 2013 and May 2016. The following information was collected: 1) baseline characteristics, 2) DMB continuation rates using the Kaplan–Meier method and reasons for stopping DMB, 3) fracture occurrence during DMB treatment, and 4) time-course of bone mineral density (BMD) [lumbar spine (LS) and total hip (TH)] and bone turnover markers (BTM) [bone-specific alkaline phosphatase (BAP), type I procollagen-N-propeptide (P1NP), type I procollagen-N-propeptide (NTX), and tartrate-resistant acid phosphatase-5b (TRACP-5b)] in 38 patients who underwent DMB treatment over a period of five years.
Results: 1) The mean age and RA duration were 69.4 years (46–86) and 17.2 years (1–49), respectively. Prednisolone and biologics were administered in 21 (32.3%) and 20 (30.8%) patients, respectively. Twenty-seven patients (41.5%) had a history of fragility fractures, and 24 patients (36.9%) had a history of vertebral fractures. Pretreatment drugs for OP were as follows: bisphosphonate in 22 patients; teriparatide, 17; none, 16; activated vitamin D, 7; and selective estrogen receptor modulator, 3.
2) Continuation rates of DMB were 96.9% at one year, 95.4% at two years, 85.8% at three years, 79.4% at four years, and 71.1% at five years. DMB treatment was terminated in 24 patients due to lack of efficacy in nine patients, death in seven patients (unknown reason in four, pneumonia in two, and senile decay in one), adverse events except death in five patients (worsening of dementia in two, brain hemorrhage in one, necrosis of jaw in one, and pneumonia in one), and other reasons in three patients.
3) Nine patients (13.8%) experienced fractures during DMB treatment; vertebral and non-vertebral fractures occurred in three and four patients, respectively. Two cases of fractures remained undefined.
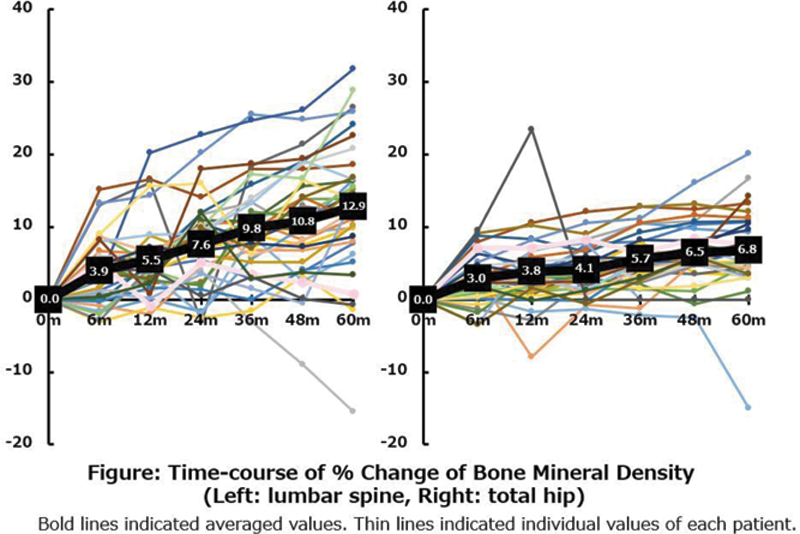
4) Both mean LS-BMD and TH-BMD significantly increased in 38 patients for whom DMB administration was continued for five years. Average percent changes of LS-BMD and TH-BMD were 3.9% and 3.0% at six months, 5.5% and 3.8% at one year, 7.6% and 4.1% at two years, 9.8% and 5.7% at three years, 10.8% and 6.5% at four years, and 12.9% and 6.8% at five years (
Conclusion: DMB treatment of RAOP proved effective and reasonably safe, and it increased BMD by a percentage similar to that observed in the FREEDOM extension trial. However, DMB administration was ceased in 13.8% of cases due to fractures and lack of efficacy. Although DMB is recommended for the treatment of RAOP, future evaluations should be conducted to predict its efficacy and determine alternative treatment strategies.
REFERENCES:
[1]Bone HG et al. Lancet Diabetes Endocrinol. 2017.
Disclosure of Interests: None declared