

Background: In a prior open-label, single-arm trial in adults with uncontrolled gout (MIRROR open-label [OL] trial), methotrexate (MTX) co-treatment with pegloticase suggested improved efficacy of pegloticase by reducing its immunogenicity. 1,2 The current randomized, controlled trial (MIRROR RCT) confirmed that pegloticase-MTX co-therapy markedly increased pegloticase response rate (response defined as serum uric acid <6 mg/dL during ≥80% of Month 6) compared to pegloticase-placebo (PBO) co-therapy (71.0% vs. 38.5%) with a decreased infusion reaction rate and no new safety signals reported.
Objectives: To evaluate systemic exposures of pegloticase and its immunogenicity in uncontrolled gout patients receiving pegloticase with and without MTX as part of the MIRROR RCT; and to determine exposure of methotrexate polyglutamate(s) (MTX-PGs) in uncontrolled gout patients through Month 6 of treatment.
Methods: In MIRROR RCT, MTX (15 mg/wk) or matching PBO was given orally 4 weeks prior to the first pegloticase dose and continued weekly, in combination with pegloticase 8 mg given intravenously every 2 weeks, over a 52-week treatment period. Pre-infusion blood samples were collected to measure MTX polyglutamates (MTX-PGs, including MTX-PG 1-5 ) in red blood cells and pre- and post-infusion serum samples were obtained to measure trough (Cmin) and peak (Cmax) concentrations of pegloticase, respectively, at multiple visits. MTX-PG and pegloticase concentrations were summarized by visit and by treatment group. Pre-infusion serum samples for anti-polyethylene glycol (PEG) antibody (Ab) measurement were also collected at multiple pre-defined time points. Anti-PEG Ab incidence and titer were summarized by visit and by treatment group.
Results: Overall, higher Cmax and Cmin of pegloticase were observed in the pegloticase + MTX group than in the pegloticase + PBO group (
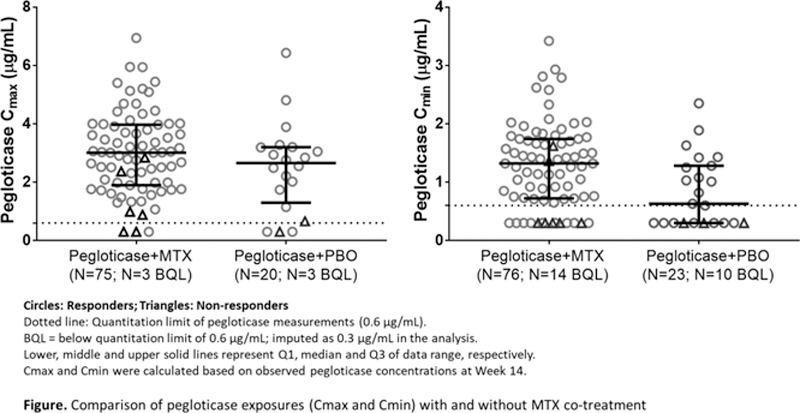
Conclusion: Pegloticase 8 mg IV every 2 weeks with MTX co-treatment (oral 15 mg weekly) reduced anti-PEG Ab incidence and resulted in higher pegloticase exposures compared to pegloticase administered with PBO, consistent with the increased clinical efficacy observed with pegloticase + MTX co-administration.
REFERENCES:
[1]Botson J, et al. J Rheumatol 2021;48:767-74
[2]Song Y, et al. Arthritis Rheum 2020;72(suppl 10)
[3]Dervieux T, et al. Ann Rheum Dis 2013;72:908-10
[4]Choi R. J Pharm Biomed Anal 2021;201:114124
Disclosure of Interests: Yan Xin Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics, Yang Song Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics, Michael E. Weinblatt Consultant of: Horizon Therapeutics, Jason Chamberlain Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics, Jennifer Zarzoso Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics, Katie Obermeyer Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics, Stephen Sainati Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics, Colleen Canavan Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics, Srini Ramanathan Shareholder of: Horizon Therapeutics, Employee of: Horizon Therapeutics