

Background: A hyperinflammatory response compatible with features of macrophage activation syndrome (MAS) contributes to this worse outcome in patients with Coronavirus Disease 2019 (COVID-19). Glucocorticoids have become the standard of care for those requiring oxygen support or mechanical ventilation. More targeted anti-inflammatory treatments with tocilizumab and anakinra have also been shown to be effective.
Objectives: More studies are being awaited to clarify the features of patients who would benefit more, and we investigated the characteristics of the surviving and dead patients who received anakinra.
Methods: The records of hospitalized adult patients between March 2020 and May 2021 in a tertiary referral center were evaluated. Diagnosis of COVID-19-related MAS was based on the expert opinion and preliminary criteria developed by our group that patients with a score of ≥45 were accepted COVID-19-related MAS. 1 Patients who received anakinra constituted the study group. Anakinra dose was determined according to the clinical and inflammatory parameters; and doses varied between daily 100-300 mg SC to 400-800 mg IV.
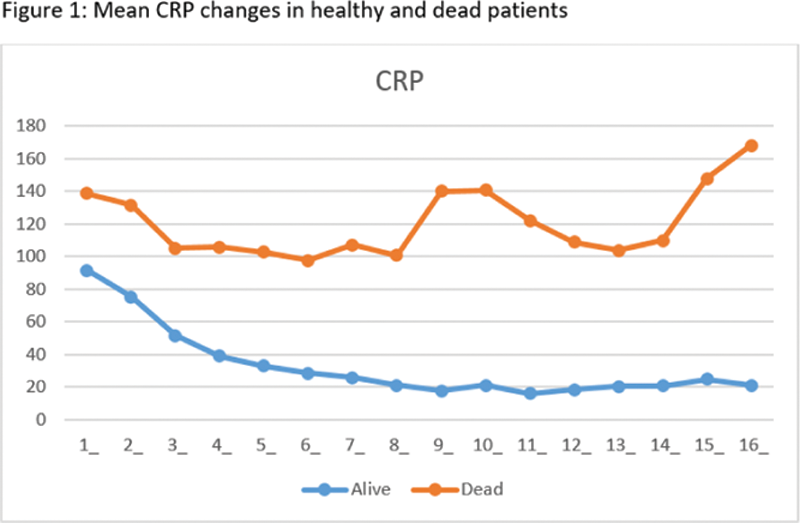
Laboratory data of surviving and died patients were comparatively analyzed by using the ANCOVA method on the relevant days (baseline, anakinra-onset day, first response to anakinra treatment, and discharge or death). The temporal variation (drug onset day-first response day, drug onset day-discharge, or death day) was evaluated using the ANOVA method. A 50% reduction of CRP compared to the anakinra start day was accepted as the first response to the treatment.
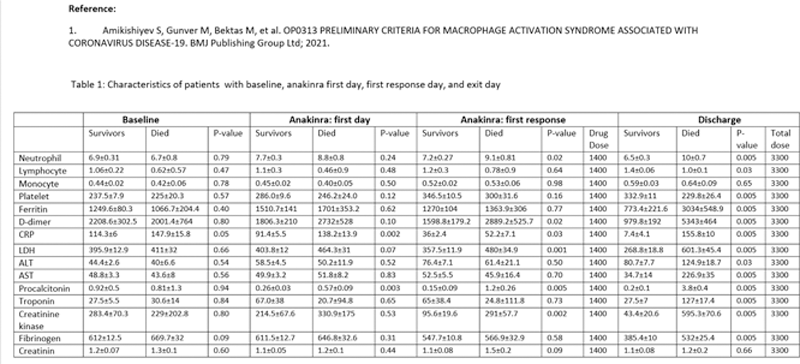
Results: Out of 1080 hospitalized patients, 218 (151 male, 67 female, mean age 60.0±14.1) who received anakinra were identified. Among them, 125 (57.3%) patients were followed in the ward, 21 (9.6%) did not need oxygen treatment during the hospitalization; 69 (31.6%) patients were followed at ICU, 40 of them were intubated, 30 (13.7%) died in ICU. Anakinra had been started in a mean of 4.8 days of hospitalization. Twenty had tocilizumab initially and then received anakinra because of ongoing inflammatory parameters. The majority (83.5%) received steroid treatment (79.5% methylprednisolone, 5% of dexamethasone), and 6 received one IV pulse 250 mg of methylprednisolone; 36 (16.5%) were followed before September 2020 and received anakinra without steroids because of the standard of care at that period. Only CRP was different between the alive and dead patients for the baseline parameters (p=0.05). On the first day of drug treatment, CRP and procalcitonin values were significantly higher in dead patients (
Conclusion: Retrospective analysis of 218 patients suggests that starting anakinra earlier in hospitalized patients may provide better results, and a decrease in CRP, ferritin, D-dimer values, as well as an increase in lymphocyte count, are associated with favorable outcomes. Increasing values of D-dimer and troponin during treatment are associated with worse outcomes, possibly indicating cardiovascular and thrombotic pathologies not responding to anakinra. Changes in the CRP values are found to help monitor the response to anakinra. Other inflammatory pathways could be targeted in those who are not responding to appropriate doses of anakinra within 5 days.
Disclosure of Interests: None declared