

Background: Previous studies proved that mRNA vaccinations against SARS CoV2 induced significant humoral responses in AIRD patients (pts). However, the humoral response was blunted in pts treated with CD20 depleting antibodies. There are limited data regarding the long-term outcome of the humoral response and the contribution of the booster vaccine, in immunosuppressed AIRD pts.
Objectives: To assess the long-term outcome of the humoral response to mRNA vaccine against SARS CoV2, in AIRD pts treated with immunomodulating drugs, and the contribution of the booster vaccination.
Methods: Consecutive pts treated at the Rheumatology Institute at Rambam Hospital who received their first SARS-CoV-2 (Pfizer) vaccine were recruited to the study, at their routine visit. The visit included AIRD activity assessment and questioning regarding vaccine side effects. We performed serology test 4-6 weeks and 24 weeks after receiving the second dose of vaccine. Pts who received the booster (3rd vaccine) were invited for serology tests 4-8 weeks afterwards. The immunomodulating treatment was not modified, either before or after the vaccination. IgG Antibodies (Ab) against SARS COV2 virus were detected using the SARS-Cov-2 IgG II Quant (Abbott) assay based on a chemiluminescent microparticle immunoassay (CMIA) on the ARCHITECT ci8200system from Abbott. This assay is measuring IgG antibodies against the spike receptor-binding domain (S-RBD) of the virus. The test was considered positive above 50 AU/ml.
Results: 262 pts (mean age(SD) 57(13), disease duration 11.2(7.4), were recruited. The cohort included 152 pts with inflammatory joint disease, 26 pts with systemic lupus erythematosus, 62 pts with other connective tissue disease and 22 pts with vasculitis; 27 % received csDMARDs only, 35% - b/tsDMARDs only, 30% - combined therapy (csDMARDs+b/tsDMARDs) and 26% received steroids. 225 pts (86%) were seropositive for IgG Ab against SARS CoV2 virus (median 2832.5 AU/ml, IQR 58-29499). 37 (14%) pts had negative tests, 23 (62.2%) of them were rituximab treated.
The IgG levels correlated with the medication used to treat the AIRD, the patients’ age but not with the type of the AIRD (
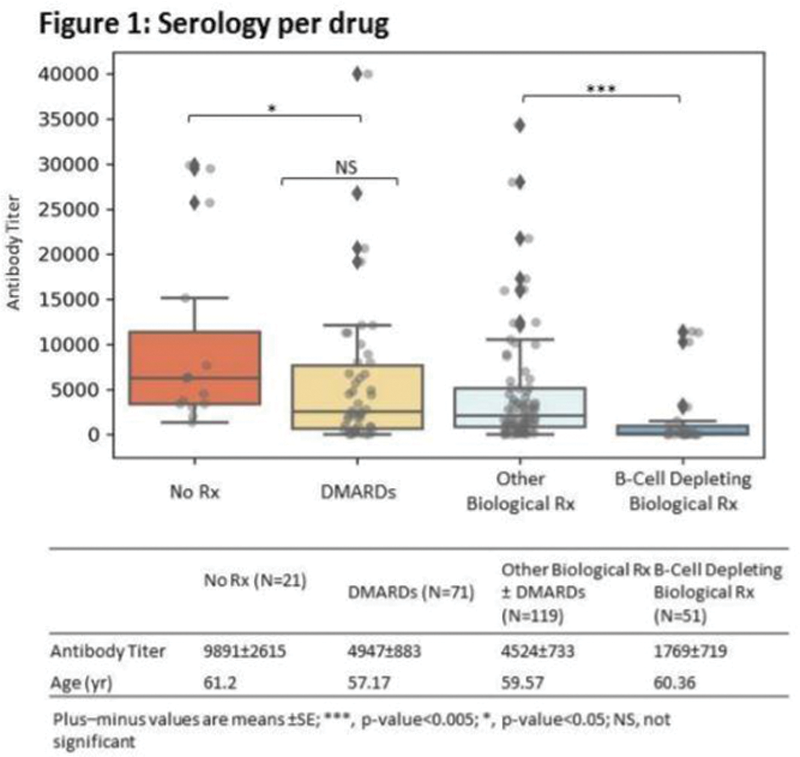
The reported side effects of the vaccine were minor (muscle sore, headache, low grade fever). The AIRD remained stable in all pts following all three vaccinations.
Conclusion: Although the vast majority of AIRD pts developed a substantial humoral response following the administration of the second dose of the Pfizer mRNA vaccine against SARS CoV2 virus, the humoral response significantly declined 24 weeks afterwards. An enhanced response was obtained after the third booster vaccination. Only minor side effects were reported and no apparent impact on AIRD activity was noted. Notably, 62% of the non-responders were treated with B cell depleting agents.
Acknowledgements: We would like to thank Mrs Tsofnat Margi and Mrs Sarit Elkouby for organisational support.
Disclosure of Interests: None declared