

Background: COVID-19 vaccines have been proven to be safe and effective in the healthy population at large. However, significant gaps remain in the evidence of their safety in patients with systemic autoimmune and inflammatory disorders (SAIDs). Patients and rheumatologists have expressed concerns regarding vaccination triggered allergic reactions, thrombogenic events, and other adverse events (ADEs) contributing to vaccine hesitancy (1)
Objectives: This study aimed to assess and compare short term COVID-19 vaccination associated ADEs in patients with SAIDs and healthy controls (HC) seven days post-vaccination, as well as between patients with SAIDs receiving different vaccines.
Methods: We developed an comprehensive, patient self-reporting electronic-survey to collect respondent demographics, SAID details, COVID-19 infection history, COVID-19 vaccination details, 7-day post vaccination adverse events and patient reported outcome measures using the PROMIS tool. After pilot testing, validation, translation into 18 languages on the online platform surveymonkey.com, and vetting by international experts, the survey was circulated in early 2021 by a multicenter study group of >110 collaborators in 94 countries. ADEs were categorized as injection site pain, minor ADEs, major ADEs, and hospitalizations. We analyzed data from the baseline survey for descriptive and intergroup comparative statistics based on data distribution and variable type (data as median, IQR).
Results: 10900 respondents [42 (30-55) years, 74% females and 45% Caucasians] were analyzed. 5,867 patients (54%) with SAIDs were compared with 5033 HCs. All respondents included in the final analysis had received a single dose of the vaccine and 69% had received 2 primary doses. Pfizer (39.8%) was the most common vaccine received, followed by Oxford/AstraZeneca (13.4%), and Covishield (10.9%). Baseline demographics differed by an older SAID population (mean age 42 vs. 33 years) and a greater female predominance (M:F= 1:4.7 vs. 1:1.8) compared to HCs.
79% had minor and only 3% had major vaccine ADEs requiring urgent medical attention overall. In adjusted analysis, among minor ADEs, abdominal pain [multivariate OR 1.6 (1.14-2.3)], dizziness [multivariate OR 1.3 (1.2-1.5)], and headache [multivariate OR 1.67 (1.3-2.2)], were more frequent in SAIDs than HCs. Overall major ADEs [multivariate OR 1.9 (1.6-2.2)], and throat closure [multivariate OR 5.7 (2.9-11.3)] were more frequent in SAIDs though absolute risk was small (0-4%) and rates of hospitalization were similarly small in both groups, with a small absolute risk (0-4%). Specific minor ADEs frequencies were different among different vaccine types, however, major ADEs and hospitalizations overall were rare (0-4%) and comparable across vaccine types in patients with SAIDs (
A. Post Vaccination ADEs in SAIDs compared to HCs. B. Proportions of post COVID-19 vaccination ADEs in SAIDs by vaccine type.
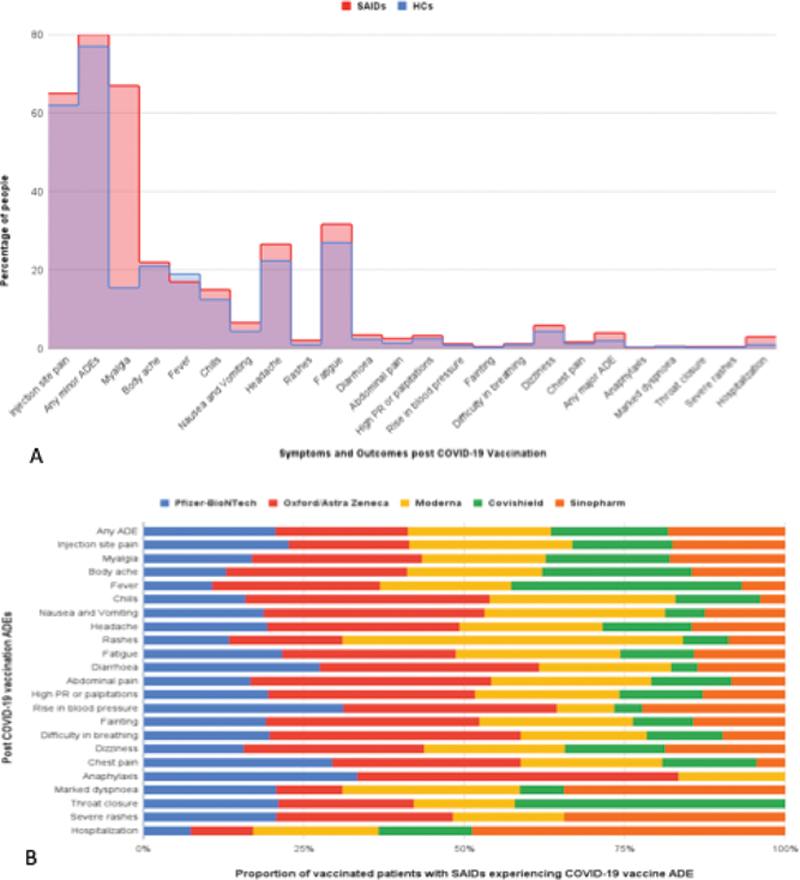
Conclusion: Vaccination against COVID-19 is relatively safe and tolerable in patients with SAIDs. Certain minor vaccine ADEs are more frequent in SAIDs than HCs in this study, though are not severe and do not require urgent medical attention. SAIDs were at a higher risk of major ADEs than HCs, though absolute risk was small, and did not lead to increased hospitalizations. There are small differences in minor ADEs between vaccine types in patients with SAIDs.
REFERENCES:
[1]Boekel L, Kummer LY, van Dam KPJ, Hooijberg F, van Kempen Z, Vogelzang EH, et al. Adverse events after first COVID-19 vaccination in patients with autoimmune diseases. Lancet Rheumatol. 2021 Aug;3(8):e542–5.
Acknowledgements: The authors thank all members of the COVAD study group for their invaluable role in the collection of data. The authors thank all respondents for filling the questionnaire. The authors thank The Myositis Association, Myositis India, Myositis UK, the Myositis Global Network, Cure JM, Cure IBM, Sjögren’s India Foundation, EULAR PARE, and various other patient support groups and organizations for their invaluable contribution in the dissemination of this survey among patients which made the data collection possible. The authors also thank all members of the COVAD study group.
Disclosure of Interests: Parikshit Sen: None declared, Naveen R: None declared, Arvind Nune: None declared, James B. Lilleker: None declared, Vishwesh Agarwal: None declared, Sinan Kardes: None declared, Minchul Kim: None declared, Jessica Day Grant/research support from: JD has received research funding from CSL Limited., Marcin Milchert: None declared, Tamer A Gheita: None declared, Babur Salim: None declared, Tsvetelina Velikova: None declared, Abraham Edgar Gracia-Ramos: None declared, Ioannis Parodis Speakers bureau: IP has received research funding and/or honoraria from Amgen, AstraZeneca, Aurinia Pharmaceuticals, Elli Lilly and Company, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Novartis and F. Hoffmann-La Roche AG., Consultant of: IP has received research funding and/or honoraria from Amgen, AstraZeneca, Aurinia Pharmaceuticals, Elli Lilly and Company, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Novartis and F. Hoffmann-La Roche AG., Grant/research support from: IP has received research funding and/or honoraria from Amgen, AstraZeneca, Aurinia Pharmaceuticals, Elli Lilly and Company, Gilead Sciences, GlaxoSmithKline, Janssen Pharmaceuticals, Novartis and F. Hoffmann-La Roche AG., Albert Selva-O’Callaghan: None declared, Elena Nikiphorou Speakers bureau: EN has received speaker honoraria/participated in advisory boards for Celltrion, Pfizer, Sanofi, Gilead, Galapagos, AbbVie, Lilly, Consultant of: EN has received speaker honoraria/participated in advisory boards for Celltrion, Pfizer, Sanofi, Gilead, Galapagos, AbbVie, Lilly, Grant/research support from: EN has received speaker honoraria/participated in advisory boards for Celltrion, Pfizer, Sanofi, Gilead, Galapagos, AbbVie, Lilly, and holds research grants from Pfizer and Lilly., Tulika Chatterjee: None declared, Ai Lyn Tan Speakers bureau: ALT has received honoraria for advisory boards and speaking for Abbvie, Gilead, Janssen, Lilly, Novartis, Pfizer, UCB., Consultant of: ALT has received honoraria for advisory boards and speaking for Abbvie, Gilead, Janssen, Lilly, Novartis, Pfizer, UCB., Grant/research support from: ALT has received honoraria for advisory boards and speaking for Abbvie, Gilead, Janssen, Lilly, Novartis, Pfizer, UCB., Lorenzo Cavagna: None declared, Miguel A Saavedra: None declared, Samuel Katsuyuki Shinjo: None declared, Nelly Ziade Speakers bureau: NZ has received speaker fees, advisory board fees and research grants from Pfizer, Roche, Abbvie, Eli Lilly, NewBridge, Sanofi-Aventis, Boehringer Ingelheim, Janssen, Pierre Fabre; none is related to this manuscript., Consultant of: NZ has received speaker fees, advisory board fees and research grants from Pfizer, Roche, Abbvie, Eli Lilly, NewBridge, Sanofi-Aventis, Boehringer Ingelheim, Janssen, Pierre Fabre; none is related to this manuscript., Grant/research support from: NZ has received speaker fees, advisory board fees and research grants from Pfizer, Roche, Abbvie, Eli Lilly, NewBridge, Sanofi-Aventis, Boehringer Ingelheim, Janssen, Pierre Fabre; none is related to this manuscript., Johannes Knitza: None declared, Masataka Kuwana: None declared, Oliver Distler Speakers bureau: OD has/had consultancy relationship with and/or has received research funding from or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the last three years: Abbvie, Acceleron, Alcimed, Amgen, AnaMar, Arxx, Baecon, Blade, Bayer, Boehringer Ingelheim, ChemomAb, Corbus, CSL Behring, Galapagos, Glenmark, GSK, Horizon (Curzion), Inventiva, iQvia, Kymera, Lupin, Medac, Medscape, Mitsubishi Tanabe, Novartis, Roche, Roivant, Sanofi, Serodapharm, Topadur and UCB. Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143)., Consultant of: OD has/had consultancy relationship with and/or has received research funding from or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the last three years: Abbvie, Acceleron, Alcimed, Amgen, AnaMar, Arxx, Baecon, Blade, Bayer, Boehringer Ingelheim, ChemomAb, Corbus, CSL Behring, Galapagos, Glenmark, GSK, Horizon (Curzion), Inventiva, iQvia, Kymera, Lupin, Medac, Medscape, Mitsubishi Tanabe, Novartis, Roche, Roivant, Sanofi, Serodapharm, Topadur and UCB. Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143)., Grant/research support from: OD has/had consultancy relationship with and/or has received research funding from or has served as a speaker for the following companies in the area of potential treatments for systemic sclerosis and its complications in the last three years: Abbvie, Acceleron, Alcimed, Amgen, AnaMar, Arxx, Baecon, Blade, Bayer, Boehringer Ingelheim, ChemomAb, Corbus, CSL Behring, Galapagos, Glenmark, GSK, Horizon (Curzion), Inventiva, iQvia, Kymera, Lupin, Medac, Medscape, Mitsubishi Tanabe, Novartis, Roche, Roivant, Sanofi, Serodapharm, Topadur and UCB. Patent issued “mir-29 for the treatment of systemic sclerosis” (US8247389, EP2331143)., Hector Chinoy Speakers bureau: HC has served as a speaker for UCB, Biogen., Consultant of: HC has received consulting fees from Novartis, Eli Lilly, Orphazyme, Astra Zeneca, Grant/research support from: HC has received grant support from Eli Lilly and UCB, Vikas Agarwal: None declared, Rohit Aggarwal Consultant of: RA has/had a consultancy relationship with and/or has received research funding from for the following companies-Bristol Myers-Squibb, Pfizer, Genentech, Octapharma, CSL Behring, Mallinckrodt, AstraZeneca, Corbus, Kezar, Kyverna, Janssen, Roivant, Boehringer Ingelheim, Argenx, Q32, Alexion, EMD Serono, Jubliant, Abbvie, Janssen, Alexion, Argenx, Q32, EMD-Serono, Boehringer Ingelheim, Roivant., Grant/research support from: RA has/had a consultancy relationship with and/or has received research funding from for the following companies-Bristol Myers-Squibb, Pfizer, Genentech, Octapharma, CSL Behring, Mallinckrodt, AstraZeneca, Corbus, Kezar, Kyverna, Janssen, Roivant, Boehringer Ingelheim, Argenx, Q32, Alexion, EMD Serono, Jubliant, Abbvie, Janssen, Alexion, Argenx, Q32, EMD-Serono, Boehringer Ingelheim, Roivant., Latika Gupta: None declared