

Background Elevated baseline (BL) C-reactive protein (CRP) levels can predict treatment response in patients (pts) with ankylosing spondylitis (AS). Tofacitinib is a Janus kinase inhibitor for the treatment of AS.
Objectives To evaluate the impact of BL CRP levels on tofacitinib efficacy and safety in pts with AS.
Methods Post hoc analysis of pooled data from placebo (PBO)-controlled, randomised, double-blind trials (NCT01786668, Phase [P]2, 16 weeks; NCT03502616, P3, 48 weeks) in pts with AS on ≥1 dose of tofacitinib or PBO (P3: PBO-treated pts switched to tofacitinib after Week [W]16), by BL CRP: normal (NML) <5 mg/L; elevated (ELV) ≥5 mg/L. Tofacitinib 5 mg twice daily (BID) efficacy was assessed to W12/W16–48 (P3). Endpoints: Assessment of SpondyloArthritis international Society (ASAS) 20/40, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) 50, AS-Disease Activity Score-CRP inactive disease (ASDAS-CRP ID), least squares mean change from BL (Δ) in nocturnal pain and Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F). Safety was assessed to W16 and W48.
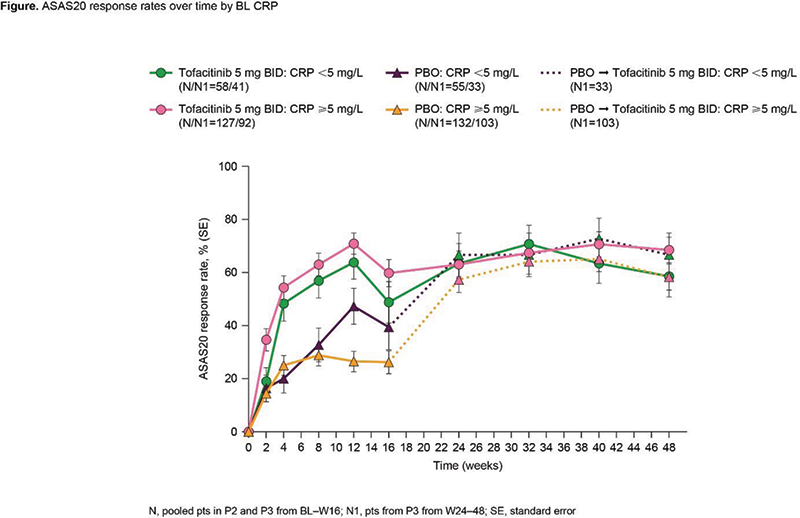
Results Of 372 pts, 30.4/69.6% had NML/ELV BL CRP. Both groups had generally similar BL characteristics; more pts with ELV CRP were male, current smokers and had prior biologic disease-modifying antirheumatic drug use. At W12, ASAS20 response was greater for tofacitinib vs PBO in both groups (Figure 1); efficacy maintained to W48. At W12, the difference in response from PBO for tofacitinib was numerically greater for ELV vs NML CRP for ASAS20 (44.7% vs 15.9%), ASAS40 (34.6% vs 17.3%), BASDAI50 (33.8% vs 15.3%), ASDAS-CRP ID (9.5% vs 8.2%), Δnocturnal spinal pain (-2.1 vs -1.4) and ΔFACIT-F (5.2 vs 3.5). For tofacitinib, rates of treatment-emergent adverse events (TEAEs) and infections to W16 trended numerically higher for tofacitinib vs PBO in pts with NML CRP, but were similar to PBO in pts with ELV CRP (Table 1). There were few serious AEs (SAEs), serious infections (SIs) or herpes zoster (HZ) across groups and no deaths. Limitations: small sample size, differences in BL characteristics.
Image/graph:
| n (%) |
To W16 | To W48 | ||||||
|---|---|---|---|---|---|---|---|---|
| Tofacitinib 5 mg BIDa | PBO | All tofacitinib 5 mg BIDb | All tofacitinibc | |||||
| NML N=58 | ELV N=127 | NML N=55 | ELV N=132 | NML N=91 | ELV N=225 | NML N=129 | ELV N=291 | |
| TEAEs | 39 (67.2) |
61 (48.0) |
27 (49.1) |
64 (48.5) |
60 (65.9) |
138 (61.3) |
80 (62.0) |
168 (57.7) |
| SAEs | 1 (1.7) |
2 (1.6) |
0 |
1 (0.8) |
5 (5.5) |
3 (1.3) |
5 (3.9) |
4 (1.4) |
| All infections | 23 (39.7) |
28 (22.0) |
13 (23.6) |
30 (22.7) |
37 (40.7) |
74 (32.9) |
46 (35.7) |
86 (29.6) |
| SIs | 1 (1.7) |
0 |
0 |
0 |
1 (1.1) |
0 |
1 (0.8) |
0 |
| HZ | 0 |
0 |
0 |
0 |
2 (2.2) |
3 (1.3) |
3 (2.3) |
4 (1.4) |
| Dis- |
1 (1.7) |
3 (2.4) |
2 (3.6) |
2 (1.5) |
4 (4.4) |
7 (3.1) |
4 (3.1) |
8 (2.7) |
aTo W12: P2; W16: P3
b To W12: P2; W48: P3
c P2: tofacitinib 2, 5 or 10 mg BID to W12; P3: 5 mg BID to W48
d Pts with events/100 pt-years
CI, confidence interval; n, number of pts with an event within risk period (on treatment); N, number of pts in safety analysis set
Conclusion Regardless of BL CRP, at W12, tofacitinib was more efficacious vs PBO; and across endpoints, the differences in response from PBO for tofacitinib were numerically greater in pts with ELV vs NML CRP. Tofacitinib safety rates were consistent with PBO in pts with ELV CRP, but trended higher for tofacitinib vs PBO in pts with NML CRP.
Acknowledgements This study was sponsored by Pfizer. Medical writing support, under the direction of the authors, was provided by Lavanyaa Manjunatha, PhD, CMC Connect, a division of IPG Health Medical Communications, and was funded by Pfizer, New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med 2022; 175: 1298-1304).
Disclosure of Interests Atul Deodhar Consultant of: AbbVie, Amgen, Aurinia, Bristol Myers Squibb, Celgene, Eli Lilly, GSK, Janssen, MoonLake, Novartis, Pfizer Inc and UCB, Grant/research support from: AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, GSK, Janssen, Novartis, Pfizer Inc and UCB, Xenofon Baraliakos Speakers bureau: AbbVie, Amgen, Chugai, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sandoz and UCB, Consultant of: AbbVie, Amgen, Chugai, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer Inc, Roche, Sandoz and UCB, Marina Magrey: None declared, Lianne S. Gensler: None declared, Amit V Thorat Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Cassandra Kinch Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Surya Pemmaraju Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Mary Jane Cadatal Shareholder of: Pfizer Inc, Employee of: Pfizer Inc, Peter Nash Speakers bureau: AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer Inc, Galapagos and GSK, Grant/research support from: AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, Pfizer Inc, Galapagos, GSK, Janssen and Novartis.
Keywords: Randomized control trial, Targeted synthetic drugs, Spondyloarthritis
DOI: 10.1136/annrheumdis-2023-eular.2118