

Background Predicting early rheumatoid arthritis (RA) from extremity MRI facilitates timely treatment, possibly preventing chronicity. Image interpretation by Artificial Intelligence (AI) may provide more accurate predictions than visual scoring.
Objectives We developed a deep learning AI-method that automatically analyzes extremity MR scans by pre- and post-processing images, in order to predict RA at an early stage.
Methods MR scans of the hands and feet from a total of 1974 patients were collected, consisting of 1247 early onset arthritis (EAC) patients, of whom 538 developed RA in two years, and 727 clinically suspect arthralgia (CSA) patients, of whom 113 developed RA. MRI scans were preprocessed automatically through background removal, slice-by-slice normalization and central slice selection. Subsequently, a self-supervised deep learning model was pre-trained to fill-in parts of the image that have been blinded by square patches (Figure 1(a), top row). Finally, after transferring the resulting weights, the model was fine-tuned to predict RA development (see Figure 1 (a) bottom row). The model was evaluated through 5-fold cross-validation and in a held-out test set (n=312 for EAC and n=146 for CSA). The model’s accuracy was evaluated with the area under the receiver operator curve (AUC). An improved class activation map was developed and applied to indicate, which areas were most important to the AI decision.
Results On the test set, the proposed model obtained a mean AUC of 0.683 in the EAC group, and 0.727 in the CSA group, using MR scans of the hands (wrist and metacarpophalangeal joints). Models trained separately on the wrists, MCPs and feet, received a mean AUC of 0.679, 0.647, 0.664, and 0.688, 0.669, 0.715, for the EAC and CSA group, respectively (see Table 1). These accuracies were close to the expert-level using RAMRIS, with reported AUCs of 0.74 and 0.69 in predicting RA in CSA [1]. According to the proposed visualization method, the deep learning models predict RA, based on very similar patterns of known (teno-)synovial inflammation and bone marrow edema (see Figure 1 (b)).
| Method: | Input | AUC |
AUC |
||
|---|---|---|---|---|---|
| Wrist | MCP | Foot | |||
| MR scans |
√ | 0.679 (±0.021) | 0.688 (±0.039) | ||
| √ | 0.647 (±0.015) | 0.669 (±0.024) | |||
| √ | 0.664 (±0.009) | 0.715 (±0.026) | |||
| √ | √ | 0.683 (±0.025) | 0.727 (±0.037) | ||
| √ | √ | √ | 0.707 (±0.016) | 0.708 (±0.068) | |
Image/graph:
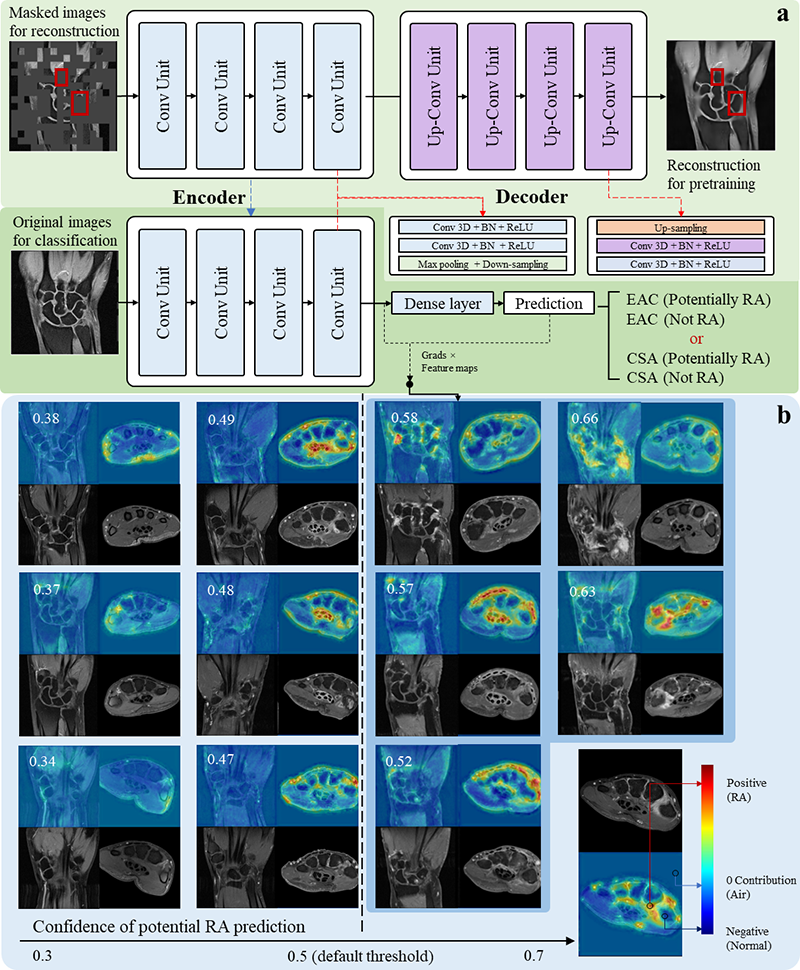
Figure 1. The overall workflow of the AI model and visualization examples from correctly-predicted samples.
Conclusion Automatic RA prediction is feasible, using AI interpretation of MRI scans. AI performed close to the level of human experts. Including MRI data from healthy controls, as used in RAMRIS-based prediction [[1]], will probably improve the AI prediction further. The new visualization method not only confirms the significance of known inflammatory features but may also point to new imaging biomarkers, giving a different perspective of understanding RA.
Reference [1] Matthijssen, XME, et al. A search to the target tissue in which RA-specific inflammation starts: a detailed MRI study to improve identification of RA-specific features in the phase of clinically suspect arthralgia. Arthritis Res Ther 21, 249 (2019).
Acknowledgements This AI-project has been funded by the Dutch Research Council (NWO) Applied and Engineering Sciences (project number 13329) and the China Scholarship Council (CSC) No.202108510012
Disclosure of Interests None Declared.
Keywords: Imaging, Rheumatoid arthritis, Artificial Intelligence
DOI: 10.1136/annrheumdis-2023-eular.3531