

Background Erosive hand osteoarthritis (OA) is a disabling disease with limited therapeutic options. Denosumab, a Receptor Activator of Nuclear Factor kappa-β Ligand inhibitor affects bone resorption and osteoclast activity.
Objectives The purpose of this clinical trial was to study the structure modifying effect of denosumab in patients with erosive hand OA, and to explore the clinical benefits and safety of subcutaneous denosumab 60 mg every 3 months versus placebo.
Methods One hundred patients with erosive hand OA were randomly allocated to placebo or denosumab in a monocentric clinical trial during 48 weeks, followed by an open-label extension phase through week 96. The primary radiographic endpoint was change in total Ghent University Scoring System (GUSS) score at week 24. GUSS (0-300) is a semi-quantitative scoring system specifically developed to combine scoring of both aspects of radiographic changes in erosive hand OA, being erosive progression (decrease of score) and signs of repair (increase of score) (1) and able to detect changes on short term [2]. The secondary endpoint was the percentage of new erosive joints at week 48. Exploratory clinical outcomes (e.g., pain, tender joint count, swollen joint count, grip strength, the Australian-Canadian Hand Osteoarthritis Index and the Functional Index for Hand Osteoarthritis) were assessed. Radiographic and clinical changes after 96 weeks of treatments were measured. Safety outcomes including (serious) adverse events, laboratory changes and changes in bone mineral density by dual-energy X-ray absorptiometry were registered. Primary efficacy analyses were performed in an intention-to-treat approach. Changes in GUSS were analysed at joint level with generalized estimating equations, accounting for within-patient clustering. Robust standard errors were used and the working correlation structure specified exchangeable. Trial registration number: EUDRACT CT 2015-003223-53.
Results 51 and 49 patients received subcutaneous administration of denosumab and placebo respectively. Total change GUSS was found statistically higher in denosumab compared to placebo at week 24 (mean change GUSS = 8.9 (95% CI: 1.0 - 16.9; p = 0.024))(Figure 1A). This difference further increased at week 48 (ΔGUSS = 14.3 (95% CI: 4.6 – 24.0; p = 0.003)). Development of new erosive joints was significantly lower in denosumab (1.8%) compared to placebo (7.0%) at week 48 (OR = 0.23 (95% CI: 0.10 to 0.50); p < 0.001)(Figure 1B). After open-label treatment through week 96, both groups continued remodelling and both pain and function significantly improved compared to baseline. Equal numbers of adverse events occurred in both groups.
Conclusion Denosumab has clear structure modifying effects in erosive hand OA compared to placebo: significantly less erosive progression occurs after 24 weeks treatment, and the treatment effect enhances after 48 weeks. Symptom improvement was observed after sustained treatment.
References
Image/graph:
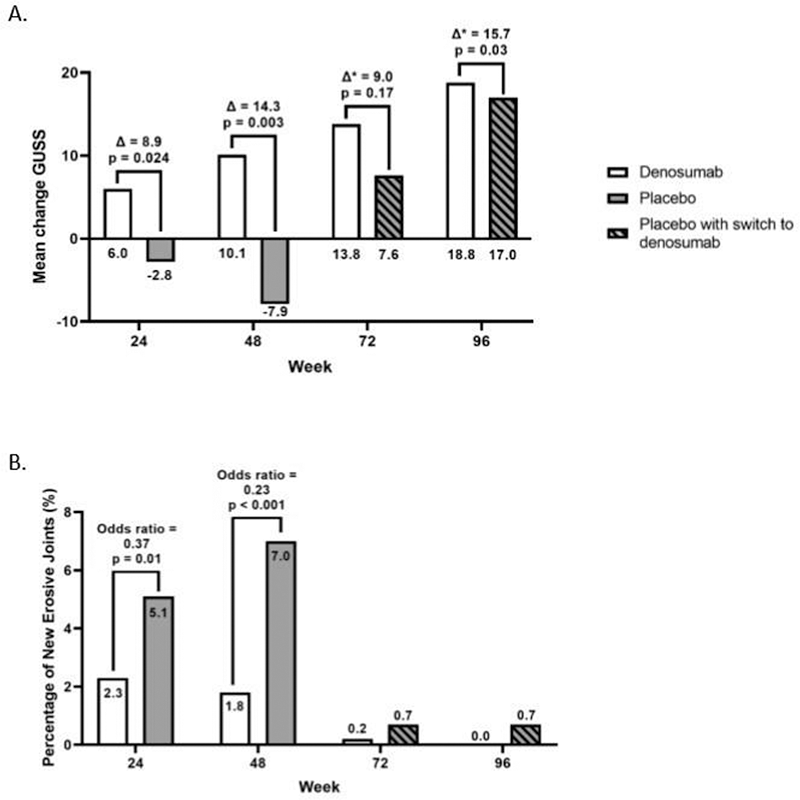
Figure 1. Radiographic changes after 48-weeks placebo-controlled phase and extension phase through 96 weeks. Panel A is showing the primary endpoint GUSS; panel B is showing the percentage of new erosive joints (secondary endpoint)
Acknowledgements: NIL.
Disclosure of Interests Ruth Wittoek Grant/research support from: ISS by Amgen; financial support to the institution, Gust Verbruggen Grant/research support from: ISS by Amgen; financial support to the institution, Tine Vanhaverbeke: None declared, Dirk Elewaut Grant/research support from: ISS by Amgen; financial support to the institution.
Keywords: Clinical Trials, Osteoarthritis
DOI: 10.1136/annrheumdis-2023-eular.3085