

Background Osteoarthritis is a major contributor to pain and disability worldwide [1]. Considering that inflammation plays an important role in the development of osteoarthritis, anti-inflammatory drugs may slow the progression of the disease [2].
Objectives To examine whether years-long use of colchicine 0.5 mg daily reduces incident total knee replacements (TKRs) and total hip replacements (THRs).
Methods We performed a post-hoc analysis of data collected in the LoDoCo2 trial to determine the time to first knee or hip replacement. A Cox proportional hazard model was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for colchicine 0.5 mg once daily as compared to placebo. Sensitivity analyses were performed by excluding patients known with gout at baseline (to avoid possible carry-over effects as colchicine is used to prevent gout attacks) or by excluding the patients who had joint surgery within the first 3 months after randomization (to avoid any bias related to planned joint surgery prior to randomization). All analyses were performed on an intention-to-treat basis.
Results Among the 5522 randomized LoDoCo2 trial participants, 2762 received colchicine and 2760 placebo during a median duration of follow-up of 28.6 months (interquartile range, 20.5 to 44.4). The mean (SD) age was 66 (8.6) years and 846 (15.3%) were female. During the trial, TKR/ THR was performed in 68 patients (2.5%) in the colchicine group and in 97 patients (3.5%) in the placebo group (HR, 0.69; 95% CI, 0.51-0.95; p = 0.02) (Table 1 and Figure 1). In a sensitivity analysis that excluded patients with gout similar results were obtained, while omitting joint replacements that took place in the first three months of follow-up yielded in an even larger rate reduction of TKR/ THR (Table 1).
| Trial cohort/subgroup | Placebo |
Colchicine |
Hazard ratio |
||
|---|---|---|---|---|---|
| No. of patients/total no. (%) | No. of events/100 person-yrs | No. of patients/total no. (%) | No. of events/100 person-yrs | ||
| Full trial population | |||||
| TKR/ THR events | 97/2760 (3.5) | 1.30 | 68/2762 (2.5) | 0.90 | 0.69 (0.51-0.95) |
| Participants with gout |
|||||
| TKR/ THR events | 89/2534 (3.5) | 1.30 | 61/2542 (2.4) | 0.88 | 0.68 (0.49-0.94) |
| TKR/ THR in the first |
|||||
| TKR/ THR events | 96/2760 (3.5) | 1.29 | 59/2762 (2.1) | 0.78 | 0.61 (0.44-0.84) |
TKR/ THR= total knee replacement/ total hip replacement
Figure 1. Cumulative incidence rates for time to first total knee/hip replacement in the full trial population.
Cumulative incidence of time to first total knee/hip replacement in participants receiving colchicine in comparison with placebo once daily. Data are shown on an enlarged y-axis.
Image/graph:
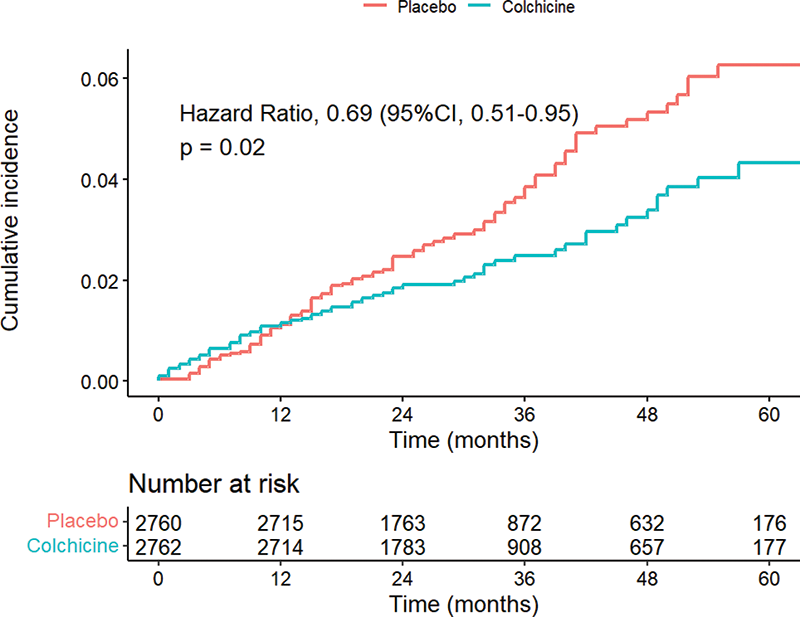
Conclusion In this post-hoc analysis of the LoDoCo2 trial, use of colchicine 0.5 mg daily was associated with a reduced risk of TKR/ THR. Further investigation of long-term therapy with colchicine to slow disease progression in osteoarthritis is warranted.
References
Acknowledgements: NIL.
Disclosure of Interests None Declared.
Keywords: Osteoarthritis, Cartilage
DOI: 10.1136/annrheumdis-2023-eular.615