

Background Potential associations between targeted therapies in patients with an inflammatory arthritis (IA)
and malignancy are a frequent concern in daily rheumatology practice. No specific framework has been proposed to evaluate the benefit/risk balance of initiating or reinitiating a targeted therapy (bDMARDs/tsDMARDs) in patients with IA and a history of cancer.
Objectives To perform a systematic literature review (SLR) to inform the task force formulating.
the EULAR Points to Consider on the initiation of targeted therapies in patients.
with IA and a history of cancer.
Methods Specific research points were defined with the task force before formulating.
the research questions with a librarian under supervision of two methodologists.
The task force agreed to focus the SLR on clinical data in patients treated with any targeted therapy for an inflammatory or autoimmune rheumatic or skin or bowel disease. All studies up to.
the 15th July 2022 were searched through Pubmed and Embase. Inclusion criteria required studies reporting on the initiation of a targeted therapy in patients with history of cancer, a control group.
of patients treated with a conventional DMARDs or healthy controls, and report of a relative risk measure (e.g. Hazard Ratio (HR)) of cancer recurrence between groups.
Two reviewers independently performed standardized article selection, data extraction, synthesis, and risk of bias assessment. The quality of the studies was graded according to the Newcastle-Ottawa quality assessment scale.
Results A total of 1555 publications were identified of which 79 articles fulfilled inclusion criteria, including.
13 published articles and 1 EULAR abstract. All studies were high quality observational data from cohorts or registries, representing 4522 patients (13030 patient-years). Most of the patients included were treated for rheumatoid arthritis. The previous cancer was a solid cancer for more than 90% of the patients. The targeted therapy evaluated was a TNF inhibitor in all the studies, and 4 studies evaluated rituximab as well.
The overall HR of cancer recurrence was 1.02 (0.83-1.26) in patients treated with a targeted therapy compared to those treated with a conventional DMARD (Figure 1). In patients treated with.
a TNF-inhibitor, the HR was 1.01 (0.86-1.18). In patients treated with rituximab, the HR.
was 1.10 (0.72-1.67). In subgroup analyses, no difference in cancer recurrence was observed if.
the targeted therapy was initiated before or after 5 years since the diagnosis of the initial cancer;
no difference in cancer recurrence was observed depending on the initial cancer type.
Conclusion The SLR informing EULAR PTC show that overall, the targeted therapies and clinical context covered by the included studies were not associated with an increased risk of cancer recurrence when compared with conventional synthetic DMARDs. This SLR also shows the lack of data for other targeted therapies, for other clinical contexts, and for other conditions than RA.
Image/graph:
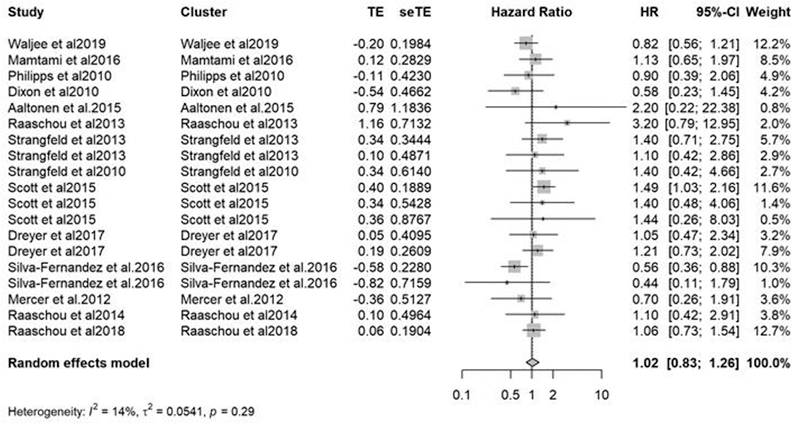
Figure 1. Relative risk of cancer between targeted therapies and csDMARDs in patients with
a history of cancer
REFERENCES:
NIL.
Acknowledgements: NIL.
Disclosure of Interests Eden Sebbag: None declared, Juan Molina Collada: None declared, Kim Lauper: None declared, Daniel Aletaha: None declared, Johan Askling: None declared, Karolina Benesova: None declared, Heidi Bertheussen: None declared, Samuel Bitoun: None declared, Ertugrul Cagri Bolek: None declared, Gerd Rüdiger Burmester: None declared, Helena Canhão: None declared, Katerina Chatzidionysiou: None declared, Jeffrey Curtis: None declared, François-Xavier Danlos: None declared, vera guimaraes: None declared, Merete Lund Hetland: None declared, Florenzo Iannone: None declared, Marie Kostine: None declared, Tue Wenzel Kragstrup Speakers bureau: Pfizer, Bristol-Myers Squibb, Eli Lilly, Novartis, UCB, and Abbvie, Consultant of: Bristol-Myers Squibb, UCB, Gilead, and Eli-Lilly, Tore K. Kvien Speakers bureau: Grünenthal, Sandoz, UCB, Consultant of: AbbVie, Amgen, Celltrion, Gilead, Novartis, Pfizer, Sandoz, UCB, Grant/research support from: AbbVie, Amgen, BMS, Galapagos, Novartis, Pfizer, UCB, Anne Regierer: None declared, Hendrik Schulze-Koops: None declared, Lucía Silva-Fernández Speakers bureau: Novartis, MSD, Sanofi, Janssen, Pfizer, Consultant of: Lilly, BMS, Abbvie, Novartis, Janssen., Zoltan Szekanecz: None declared, Maya H Buch: None declared, Axel Finckh Speakers bureau: AbbVie, BMS, Pfizer, Eli-Lilly, Sandoz, Consultant of: AbbVie, Novartis, Pfizer, MSD, Lilly, Grant/research support from: AbbVie, BMS, Galapagos, Lilly, Pfize, Jacques-Eric Gottenberg Consultant of: Abbvie, BMS, Galapagos, Gilead, Jannsen, Lilly, Roche Chugai, Sanofi, Pfizer, UCB, Grant/research support from: Abbvie, BMS, Pfizer.
Keywords: Comorbidities, bDMARD, Malignancy
DOI: 10.1136/annrheumdis-2023-eular.4884