

Background Pain is commonly cited by patients with psoriatic arthritis (PsA) as affecting their daily activities and quality of life. Pain signaling involves a variety of cytokines, such as interleukin (IL)-17, interferon (IFN)-γ, and IL-6. TYK2 mediates signaling of key cytokines, such as IL-23, which is upstream of IL-17, involved in PsA pathogenesis. Deucravacitinib (DEUC) is a first-in-class, oral, selective, allosteric inhibitor of TYK2, approved in multiple countries for the treatment of adults with plaque psoriasis [1,2]. DEUC was efficacious vs placebo (PBO) in a phase 2 trial in patients with active PsA, and cytokine levels were reduced with DEUC treatment vs PBO, including IL-17A and other cytokines reflecting downstream anti-inflammatory effects of TYK2 inhibition, including IL-6 and tumor necrosis factor alpha [3,4].
Objectives To characterize the effect of DEUC on pain across different instruments, and alignment across pain instruments, in patients in the phase 2 PsA trial.
Methods Patients with PsA (N=203) were randomized 1:1:1 to PBO, DEUC 6 mg once daily (QD), or DEUC 12 mg QD. Three instruments were used to assess pain up to week 16: (1) Patient Global Assessment of Pain visual analog scale (Pain VAS), scored from 0-100; (2) Psoriatic Arthritis Impact of Disease (PsAID) Pain instrument, scored from 0-10; and (3) 36-Item Short-Form Health Survey (SF-36) Bodily Pain question, which asks patients to rate their pain on scale of 1-6 ranging from “none” to “very severe.” Mean change from baseline (BL) in pain scale scores, the proportion of patients who reported meaningful improvements in pain, and Pearson’s correlation between pain scales (Pain VAS and PsAID Pain) and disease efficacy measures were evaluated.
Results BL mean Pain VAS score was 64.1 and BL mean PsAID Pain score was 6.4, and scores were generally similar across treatment groups. Percentages of patients who reported improvements in Pain VAS (Figure 1) and PsAID Pain were consistently greater with DEUC treatment compared with PBO, with improvements in pain being similar between males and females across instruments. The pain assessments correlated with one another both at baseline (Table 1) and over time through week 16, with some divergent responses to pain questions also being observed. At baseline, both assessments of pain strongly correlated with Psoriatic Arthritis Disease Activity Score (PASDAS) and with Patient Global Assessment of Disease Activity (PtGA) (Table 1).
Conclusion A higher proportion of patients with PsA treated with DEUC reported clinically meaningful improvements in pain compared with PBO. Patient-reported pain was overall well-correlated across instruments; however, some divergence was also observed.
References
Image/graph:
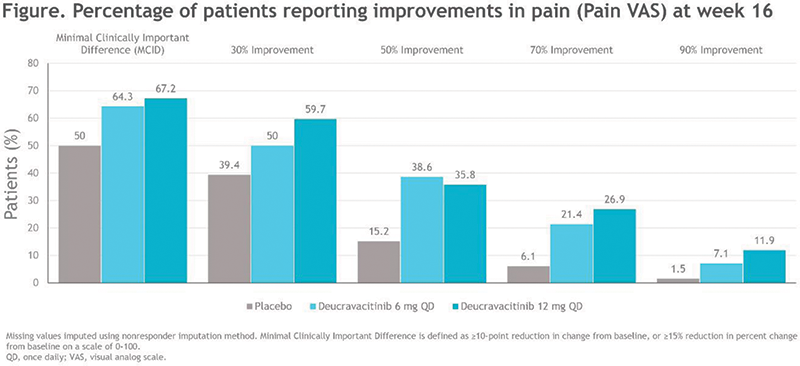
| Pain VAS | PsAID Pain | |
|---|---|---|
| Pain VAS | – | 0.751 |
| PsAID Pain | 0.751 | – |
| PASDAS | 0.618 | 0.578 |
| PtGA | 0.653 | 0.583 |
| DAPSA | 0.495 | 0.483 |
| DAS28 | 0.466 | 0.475 |
| HAQ-DI | 0.423 | 0.498 |
| TJC | 0.351 | 0.386 |
| PGA | 0.367 | 0.286 |
| SJC | 0.305 | 0.291 |
| CRP | 0.190 | 0.220 |
The strength of the Pearson’s correlation coefficient is coded by color, with green being a strong correlation (0.5-1.0), yellow is medium (0.3-0.5), and blue is weak (0.1-0.3). CRP, C-reactive protein; DAPSA, Disease Activity in Psoriatic Arthritis; DAS28, Disease Activity Score – 28 joint; HAQ-DI, Health Assessment Questionnaire – Disability Index; PASDAS, Psoriatic Arthritis Disease Activity Score; PGA, Physician Global Assessment of Disease Activity; PsAID, Psoriatic Arthritis Impact of Disease; PtGA, Patient Global Assessment of Disease Activity; SJC, swollen joint count; TJC, tender joint count; VAS, visual analog scale.
Acknowledgements This study was sponsored by Bristol Myers Squibb.
Disclosure of Interests Philip J Mease Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, SUN Pharma, and UCB, Grant/research support from: AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Sun Pharma, and UCB, Lihi Eder Consultant of: AbbVie, Novartis, UCB, Pfizer, Eli Lilly, Janssen, Grant/research support from: AbbVie, Novartis, UCB, Pfizer, Sandoz, Fresenius Kabi, Eli Lilly, Janssen, Alexis Ogdie-Beatty Consultant of: AbbVie, Amgen, BMS, Celgene, Corrona, Gilead, Janssen, Lilly, Novartis, Pfizer, and UCB, Grant/research support from: Pfizer to Penn, Novartis to Penn, Amgen to Forward/NDB, Atul Deodhar Consultant of: AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galapagos, GlaxoSmithKline, Janssen, Novartis, Pfizer, and UCB, Grant/research support from: AbbVie, Eli Lilly, GlaxoSmithKline, Novartis, Pfizer, and UCB, Subhashis Banerjee Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb, Miroslawa Nowak Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb, Jiyoon Choi Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb, Thomas Lehman Shareholder of: Bristol Myers Squibb, Employee of: Bristol Myers Squibb, Vibeke Strand Consultant of: AbbVie, Amgen, Arena, AstraZeneca, Bayer, Biosplice, Bioventus, Blackrock, BMS, Boehringer Ingelheim, Celltrion, Chemocentryx, EMD Serono, Equilium, Eupraxia, Flexion, Galapagos, Genentech/Roche, Gilead, GSK, Horizon, Ichnos, Inmedix, Janssen, Kiniksa, Kypha, Lilly, Merck, MiMedx, Novartis, Pfizer, Regeneron, Rheos, Samsung, Sandoz, Sanofi, Scipher, Servier, Setpoint, Spherix, Tonix, and UCB.
Keywords: Targeted synthetic drugs, Pain, Psoriatic arthritis
DOI: 10.1136/annrheumdis-2023-eular.1691