

Background: Antiphospholipid Syndrome (APS) is an autoimmune pro-thrombotic condition characterized by thrombotic and gestational complications combined with the persistence of antiphospholipid antibodies (aPL). Due to the heterogeneous clinical presentation of APS, several efforts have been expended to establish biomarkers for assessing individual thrombotic risk, including epigenetic biomarkers. MicroRNAs (miRNAs) are single-stranded, non-coding RNA molecules that control post-transcriptional gene expression regulation. In the context of APS, miRNAs may have an impact as modulators of the autoimmune response and hemostatic disturbances, leading to a procoagulant state and clinical thrombosis, especially through the regulation of tissue factor expression. However, evidence correlating the prevalence of dysregulated miRNAs and thrombotic risk stratification in APS is still scarce.
Objectives: The aim of this study is to evaluate which miRNAs associated with APS are also associated with the risk of thrombosis.
Methods: This is a systemic review study that involved a comprehensive analysis of clinical studies evaluating miRNAs as potential biomarkers in thrombotic APS and in venous or arterial thrombosis in non-APS patients. We conducted a comprehensive search across multiple scientific databases using the terms “MicroRNAs AND Biomarkers AND (“Antiphospholipid syndrome” OR “Thrombosis” OR “Lupus erythematosus systemic”). Two blinded reviewers analyzed the possible inclusion of all returned references, firstly by evaluating the abstracts (step 1) and subsequently the full text (step 2). Any conflicts were resolved by a third reviewer. Non-clinical studies, duplicate references, systematic reviews, and references describing miRNAs as biomarkers for clinical conditions not related to thrombotic events or focusing on other non-coding RNAs were excluded.
Results: Out of 1020 unique references available until 2022, 44 met the inclusion criteria of this study and were analyzed (Figure 1). Among these, eight studies demonstrated 39 miRNAs expressed differently in patients with thrombotic APS compared to non-APS patients, while 16 studies showed 45 dysregulated miRNAs in patients with non-APS-related arterial thrombosis, and 20 studies described 48 differentially expressed miRNAs in non-APS-venous thrombosis. Two distinct groups of miRNAs were identified. First, a group of miRNAs related to both thrombotic APS and non-APS arterial thrombosis (miRs- 124-3p; 125b-5p; 125a-5p; 17-5p). Another group was related to thrombotic APS and non-APS venous thrombosis (miRs-106a-5p; 146b-5p; 15a-5p; 222-3p, 451a). Only miR-126a-3p was found to be differentially expressed in thrombotic APS and non-APS arterial and venous thrombosis (see Figure 2).
Flowchart of the study
Differentially expressed microRNAs according to the type of thrombosis and the diagnosis of APS.
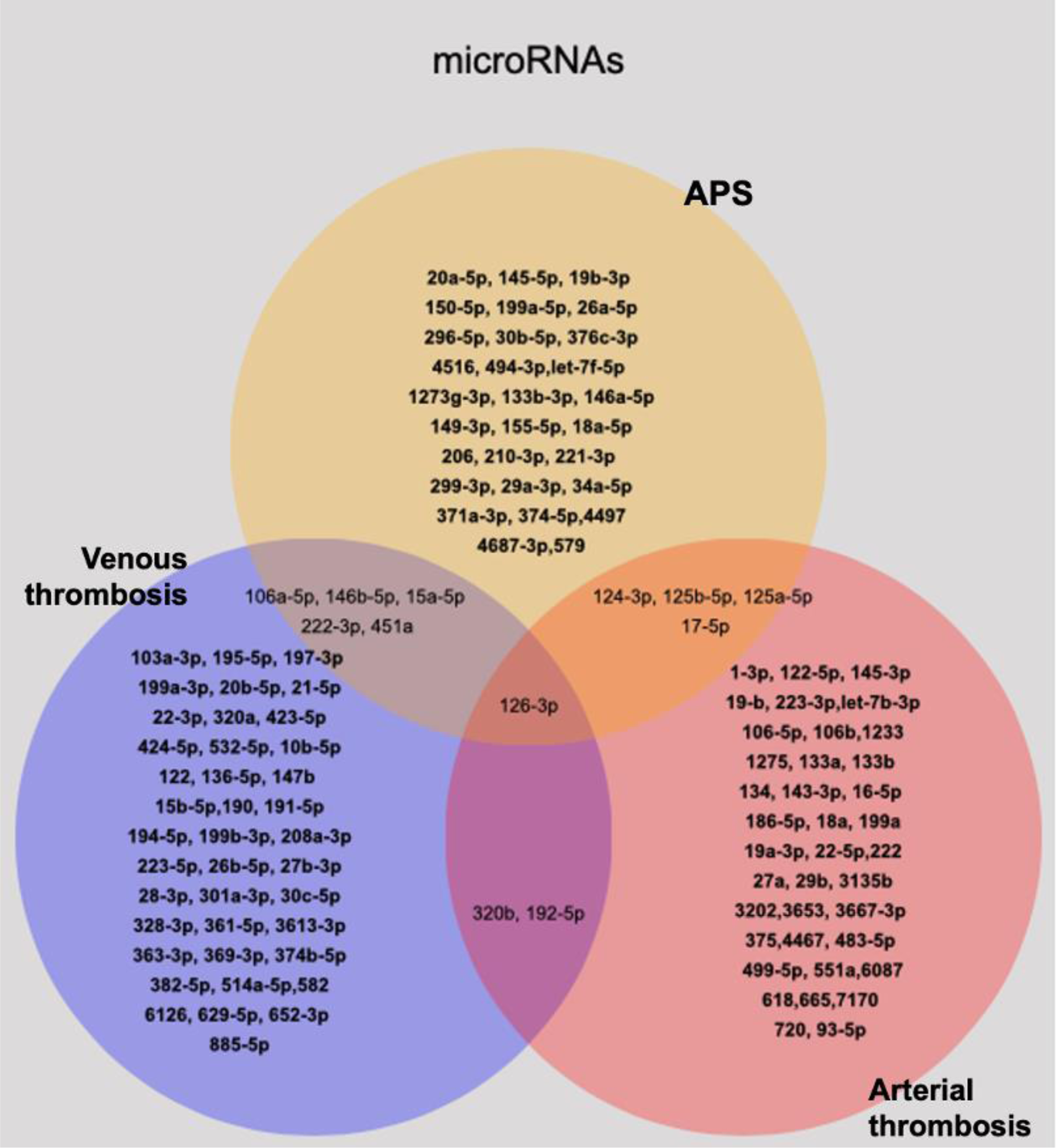
Conclusion: In systemic review of the medical literature, we identified 10 mRNAs differently expressed in APS that could form a biomarker of the thrombotic risk in the disease. These results encourage the validation of these biomarkers as laboratory criteria for APS risk classification.
REFERENCES: NIL.
Acknowledgements: São Paulo Research Foundation- FAPESP (2020/07922-4).
Disclosure of Interests: None declared.