

Background: The new classification criteria for antiphospholipid antibody syndrome (APS) have introduced many novelties, such as the presence of an entry criterion, the use of a scoring system, the introduction of previously “extra-criteria” manifestations, the weighting of thrombotic events according to thromboembolic and cardiovascular risk factors and the redefinition of obstetric APS (OAPS).
Objectives: The aim of this study is to apply the latest classification criteria to a monocentric cohort of patients with diagnosis of APS and to compare it with the previous criteria.
Methods: We retrospectively collected data from patients with clinical diagnosis of APS from the Lupus Clinic of Sapienza University of Rome and applied the new classification criteria and the previous 2006 “Sapporo” criteria.
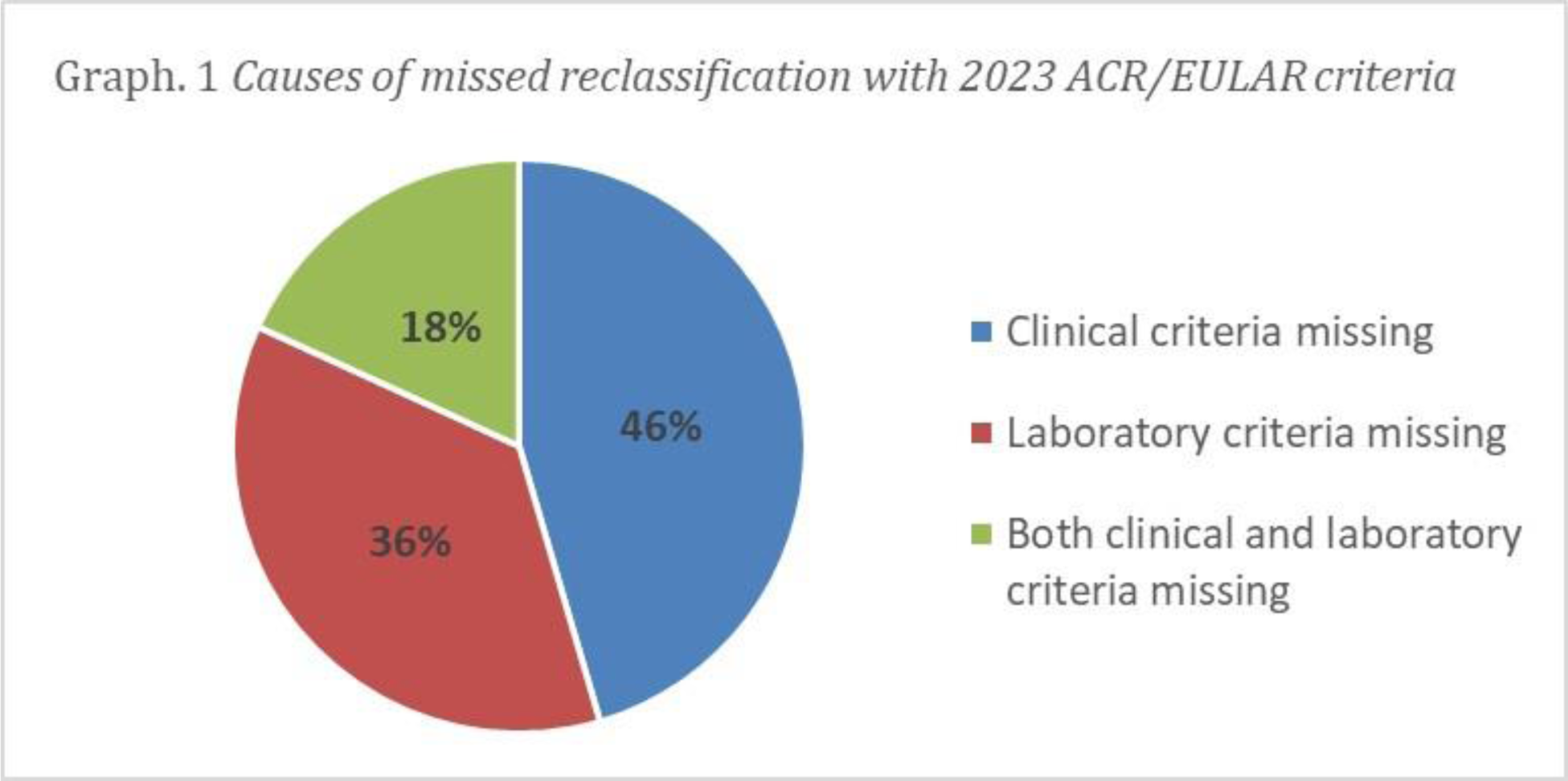
Results: In our cohort of 165 APS patients, 139 could be classified according to Sapporo criteria, while 106 patients fulfilled the new classification criteria. Among the 33 patients that could not be reclassified by the new criteria, 21 patients did not meet clinical domains (8 thrombotic APS patients did not reach enough points due to a high thromboembolic/cardiovascular risk and 13 OAPS patients due to the presence of poliabortivity or fetal death alone), while 18 lacked laboratory domains due to only IgM aPL positivity (Graph 1).
Conclusion: In our cohort, the application of the new APS classification criteria identifies a smaller number of patients compared to the previous ones, confirming the reduced sensitivity of the former. The new criteria may lead to the exclusion of patients with specific clinical and laboratory characteristics, that require larger studies to be assessed.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.