

Background: Patients on immunosuppressive therapies have diminished humoral vaccine responses to SARS-CoV-2 vaccines. Cellular immunity is crucial for long-term protection against severe COVID-19. Interferon gamma release assay (IGRA) SARS-CoV-2 tests are now commercially available for evaluation of cellular responses in clinical practice. Knowledge on the durability of T cell responses is important to plan further booster doses.
Objectives: To evaluate durability of cellular and humoral responses following a 5 th vaccine dose.
Methods: The observational, prospective Nor-VaC study examines vaccine responses in patients with inflammatory joint diseases on immunosuppressive therapies. The present analyses include a subgroup of patients eligible for a 5 th vaccine dose with samples available for analyses. CD4, plus combined CD4 and CD8 T-cell responses (CD4;CD8), were determined by interferon gamma release assay (IGRA) Elecsys QuantiFERON SARS-CoV-2, along with spike IgG antibodies measured by Elecsys Anti-SARS-CoV 2 S immunoassay (Roche), prior to and 2-4 weeks and 6 months following the 5 th dose. T-cell responses were defined as negative (<0.030 IU/mL), uncertain (0.030-0.050 IU/mL) or positive (>0.050 IU/mL). Serologic poor responders were defined by response <2000 IU/mL. SARS-CoV-2 infections were defined as a positive PCR or antigen test, self-reported by patients.
Results: Between Nov 10, 2022, and Apr 19, 2023, a total of 63 patients (37 rheumatoid arthritis, 15 psoriatic arthritis, 11 spondyloarthritis) using TNFi a (n=28), methotrexate (n=20) or other DMARD b (n=15), median age 63 (IQR 55-69), 65 % female, were included. Patients received a 5th bivalent BA.1 (13%) or BA.4/5 (79%) vaccine dose. Five patients were lost to follow-up after the pre-vaccination sample.
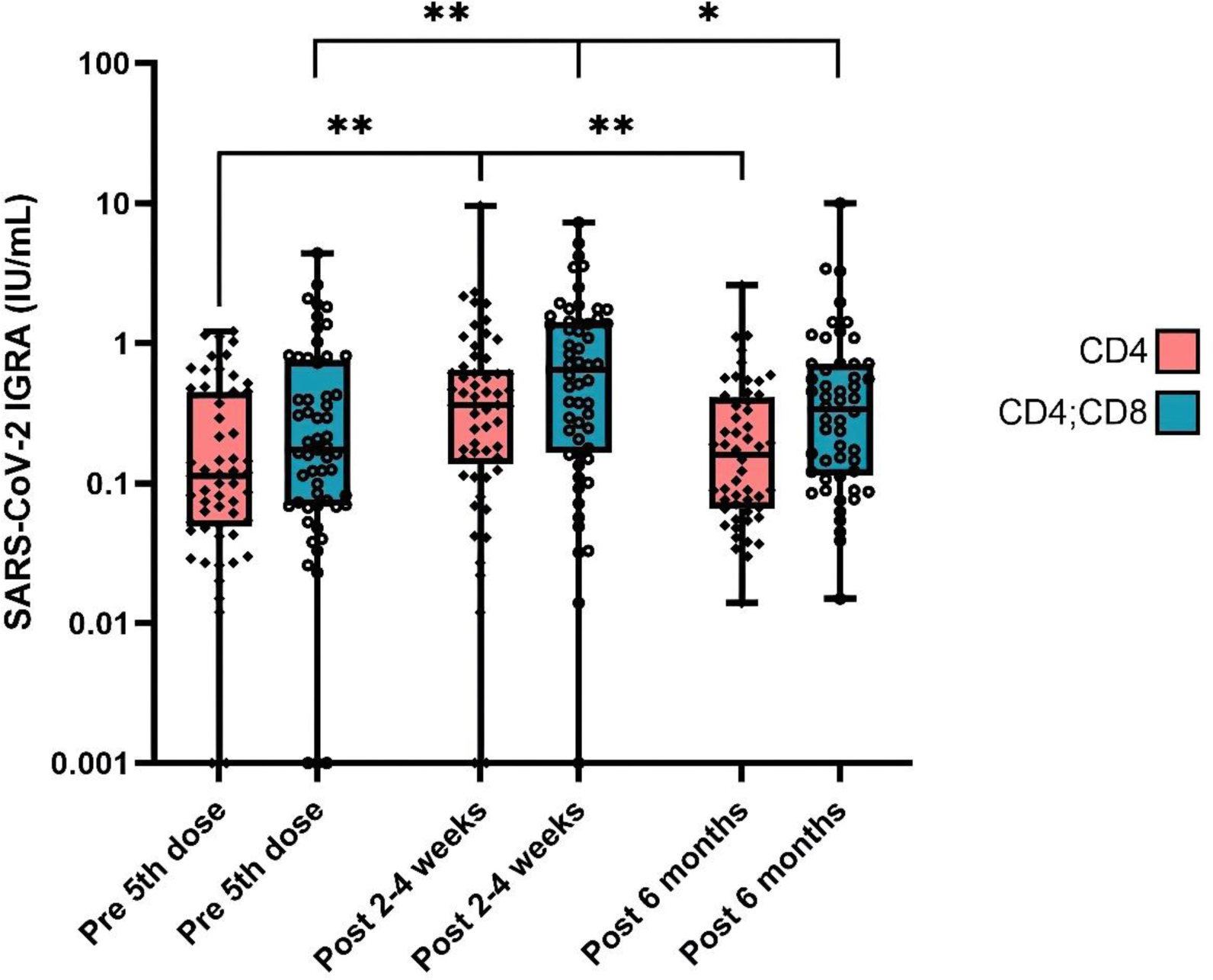
2-4 weeks after the 5th dose, there was an increase in both CD4 (p=0.001) and CD4;CD8 T cell activity (p=0.002) compared to prior 5th dose (Table 1, Figure 1), with subsequent waning in activity after 6 months for both CD4 (p=0.006) and CD4;CD8 (p=0.044). (Table 1, Figure 1) Prior to the 5th vaccine dose vs 2-4 weeks post-vaccination, 18% (10/57) vs 9% (5/57) had negative CD4-responses, and 9 % (5/57) vs 4% (2/56) had negative CD4;CD8 T cell responses. In comparison, 6 months post-vaccination 4% (2/52) had negative CD4 and 2% (1/52) had negative CD4;CD8 T cell responses. This reduction of patients with negative CD4 and CD4;CD8 T cell responses after six months may be attributed to undergoing SARS-CoV-2 infection prior to the 6-month sample. Overall, 6 patients (10%) reported a SARS-CoV-2 infection between the 5th vaccine dose and the 6-month sample. In addition, subclinical infections may have occurred. Anti-spike antibody levels increased 2-4 weeks post- vs pre-vaccination, p=0.002, with a decrease at 6 months compared to 2-4 weeks post-vaccination (p=0.003). (Table 1) Among the serologic poor responders (n=7), two had negative CD4 responses following a 5th vaccine dose, but all showed positive or intermediate CD4;CD8 responses.
Conclusion: Despite a decline in both cellular and humoral immunity over the observation period, most patients had detectable T cell activity and high antibody levels six months after receiving a 5 th vaccine dose of the updated SARS-CoV-2 vaccine. Individualised testing of cellular immunity in patients with poor serologic responses to vaccines could be valuable to determine timing of future vaccine boosters.
Table 1. Cellular and humoral immune responses pre-, 2-4 weeks- and 6 months post-vaccination.

a Tumour necrosis factor inhibitors in mono- or combination therapy
b Rituximab, interleukin inhibitors, janus kinase inhibitors, abatacept
Cellular responses measured by SARS-CoV-2 IGRA pre-, 2-4 weeks, and 6 months post-5 th vaccine dose. *<0.05, **<0.01.
REFERENCES: NIL.
Acknowledgements: We thank the patients who have participated in the Norwegian study of vaccine response to COVID-19. We thank the patient representatives in the study group, Kristin Isabella Kirkengen Espe and Roger Thoresen. We thank all study personnel, laboratory personnel, and other staff at the clinical departments involved, particularly Margareth Sveinsson, May Britt Solem, and Kjetil Bergsmark.
Disclosure of Interests: Hilde S. Ørbo: None declared, Ingrid Jyssum: None declared, Anne Therese Tveter: None declared, Andreas Lind: None declared, Veselka P. Dimova-Svetoslavova: None declared, Ingrid E. Christensen: None declared, Joe Sexton: None declared, Kristin Hammersbøen Bjørlykke Janssen-Cilag, Tore K. Kvien Grünenthal, Janssen, Sandoz, AbbVie, Gilead, Janssen, Novartis, Pfizer, Sandoz, UCB, AbbVie, BMS, Galapagos, Novartis, Pfizer, UCB, Espen A. Haavardsholm Pfizer, UCB, Novartis, Abbvie, Pfizer, Eli Lilly, Kristin Kaasen Jørgensen Bristol-Myers Squibb, Roche, Sella Aarrestad Provan: None declared, Silje Watterdal Syversen: None declared, Guro Løvik Goll AbbVie/Abbott, Galapagos, Pfizer, UCB.