

Background: After Sars-CoV-2 outbreak, considering its high mortality and diffusiveness, a mass worldwide vaccination campaign was put in place. Some groups of patients were prioritized in this process. Among them, Systemic Lupus Erythematosus (SLE) ones were considered at high risk of death from COVID-19, especially due to the immunosuppressive (Is) drugs administered. Moreover, at vaccines release there was a complete absence of efficacy and safety data in patients with autoimmune diseases, since phase III trials of most vaccines excluded subjects on Is drugs.
Objectives: To assess the impact of Is drugs on BNT162b2 mRNA vaccine’s immunogenicity and safety in SLE patients.
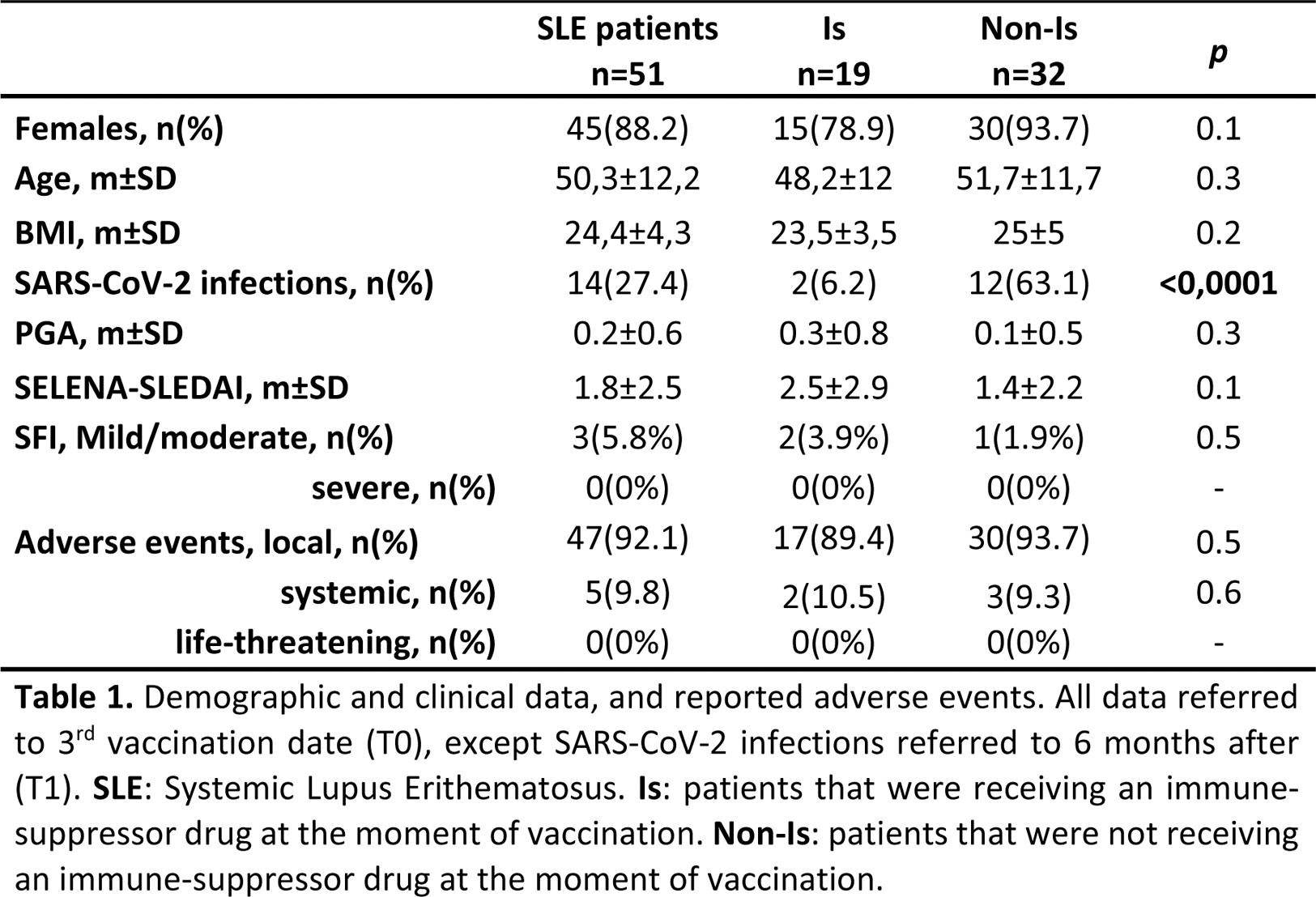
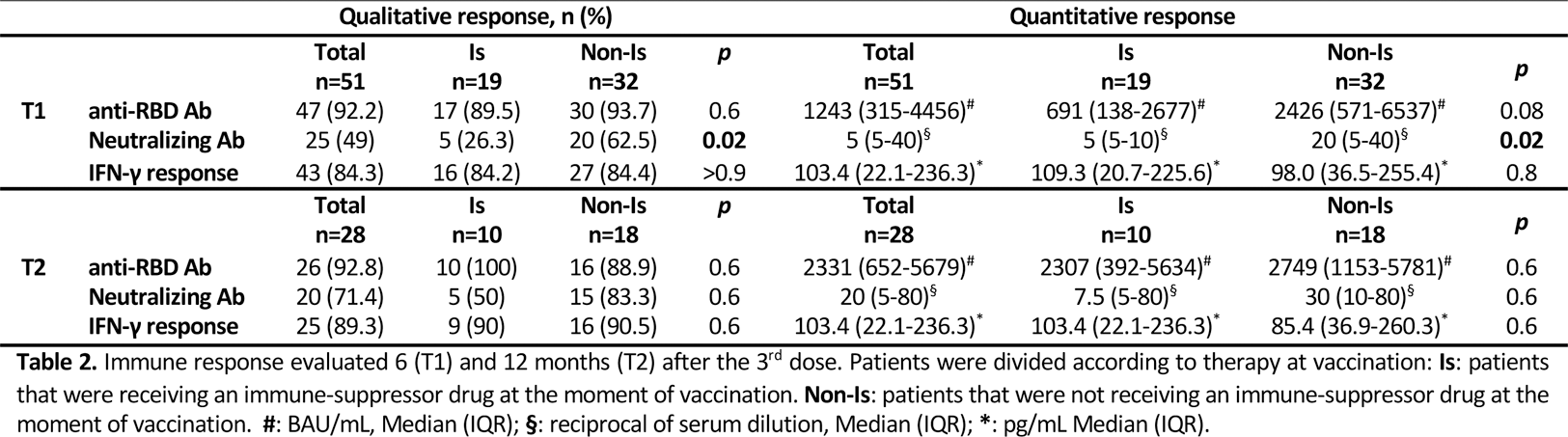
Methods: This is a prospective real-life study on consecutively enrolled SLE patients conducted between March 2022 and January 2023. Inclusion criteria: age > 18 and < 70 years, SLE diagnosis (according to EULAR/ACR 2019 criteria) prior to SarS-CoV-2 outbreak, vaccination with 3 doses of BNT162b2 vaccine. Exclusion criteria: high disease activity (SLEDAI-2K >10) or ongoing disease flare at inclusion, treatment with Rituximab or intravenous Immunoglobulins in the last 12 or 2 months respectively. Patients were enrolled 7 days after their 3rd vaccine shot (T0) when demographic and clinical data as well as ongoing therapies (Table 1) were collected, along with eventual adverse events. 6 months (T1) and 12 months (T2) after their 3rd dose, humoral and cellular immune responses were evaluated (both qualitatively and quantitatively). The former analysing anti-receptor binding domain (RBD) IgG antibodies (Ab) with an immunoassay analyser and neutralizing (Neu) Ab through a microneutralization assay. The latter was evaluated analysing interferon (IFN)γ release using an enzyme-linked immunosorbent assay after stimulating whole blood with Sars-CoV-2 peptides.
Then, patients were divided in two groups based on therapy at vaccination:
Is: patients receiving an Is drug, including prednisone at dosage higher than 5mg/day, regardless of concomitant Hydroxychloroquine (HCQ) therapy
Non-Is: patients not receiving any Is drugs (i.e. HCQ or prednisone (lower than 5mg/day) alone or in combination; patients not receiving any drug for SLE)
The two groups were then compared to establish the influence of eventual Is drugs on immune response after a full vaccination cycle.
Results: 51 patients were enrolled at T0 (Table 1). Comparing the two groups, no differences emerged for either demographic or clinical parameters, apart for a higher number of SarS-CoV-2 infections in the Non-Is group (p<0.0001). Equally, local and systemic adverse events did not differ among the groups and no life-threatening events were registered. Regarding immune response (Table 2), all patients were available at T1 follow-up visit. 47 (92.2%) showed anti-RBD Ab and 25 (49%) Neu Ab; cellular response was detectable in 43 (84.3%) subjects. The only significant difference regarded Neu Ab, which resulted higher in the Non-Is group both qualitatively (p=0.02) and quantitatively (p=0.02). 28 patients (54.9%) returned at T2. Anti-RBD Ab were still present in 26 patients (92.8%), while Neu Ab in 20 (71.4%). Cellular response was detectable in 25 (89.3%) patients. Both for humoral and cellular response, no significant difference was detectable comparing the two groups Is and Non-Is patients. Lastly, a significant correlation was found between anti-RBD Ab and both Neu Ab and cellular response (data not shown) both at T1 (p<0.001 and =0.002, respectively) and T2 (p<0.001 and =0.005, respectively).
Conclusion: Anti-SarS-CoV-2 BNT162b2 mRNA vaccine can induce a persistent immune response in SLE patients while being safe and well tolerated. In fact, even in patients on Is therapy, immune response can be retrieved 1 year after its administration – without being influenced by Is therapies at vaccination. Indeed, Is therapy reduced Neu Ab production without otherwise interfering with the other immune response components. Moreover, whilst no life-threatening events were registered, Is therapies were not associated with adverse events onset.
REFERENCES: [1] Aiello A. (2021) Int J Infect Dis, 106:338.
[2] Tan SY. (2023) Rheumatology (Oxford), 62(5): 1757.
Acknowledgements: NIL.
Disclosure of Interests: None declared.