

Background: Inflammatory arthritis (IA) is a common adverse event following treatment with immune checkpoint inhibitors. The absence of antibodies in IA and clinical disparities between IA and rheumatoid arthritis (RA) imply disease heterogeneity and distinct underlying immunopathological mechanisms, which remain elusive.
Objectives: We aimed to comprehensively characterize the immunophenotype of synovial fluid and peripheral blood of patients with active PD-1-IA, patients with PD-1-IA in remission, patients with seropositive RA and healthy controls (HCs).
Methods: We profile CD45 + hematopoietic cells from the peripheral blood or synovial fluid (SF) of patients with PD-1-induced IA (PD-1-IA) or RA using single-cell RNA sequencing and validate using Flow cytometry, ELISA, and Chemotaxis assay.
Results: We report the predominant expansion of IL1B hi myeloid cells with enhanced NLRP3 inflammasome activity, in both the SF and peripheral blood of patients with PD-1-IA, but not RA. IL1B hi macrophages in the SF of PD-1-IA shared similar inflammatory signatures and might originate from IL1B hi monocytes in the peripheral blood. We also observed a significant accumulation of an exhausted CD8 + T-cell population in the SF of PD-1-IA patients. IL1B hi myeloid cells orchestrated cell communication with exhausted CD8 + T cells possibly via the CCR1-CCL5/CCL3 and CXCL10-CXCR3 axes.
Conclusion: Collectively, these results provide evidence of different cellular and molecular pathways in PD-1-IA and RA and highlight the importance of IL1B hi macrophages as a therapeutic target in PD-1-IA.
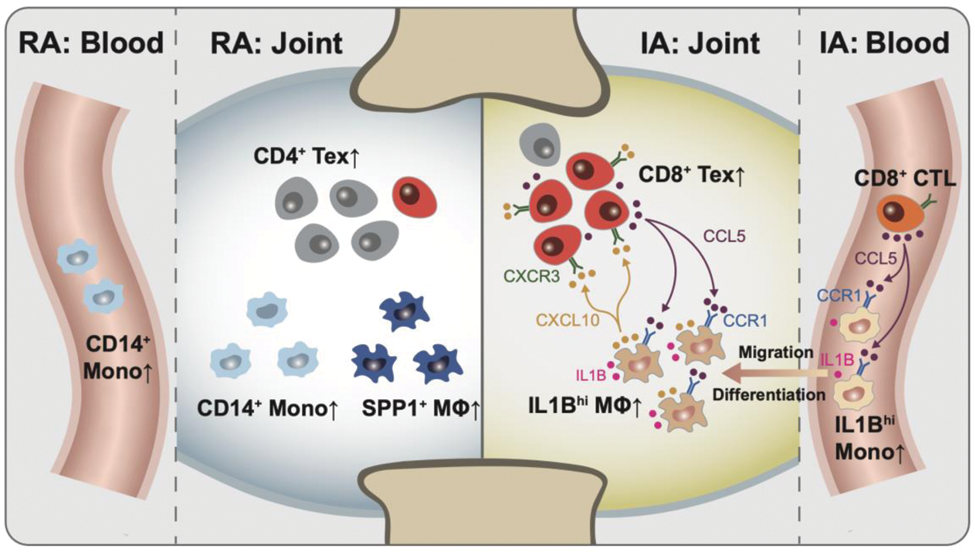
Graphical summary of this study. In IA, synovial IL1B hi macrophages, likely originating from peripheral IL1Bhi CD14+ monocytes, communicate with exhausted CD8 + T cells through the CCR1-CCL5/CCL3 and CXCL10-CXCR3 axes (right). This interaction may play a key role in PD-1-IA pathogenesis but not in RA (left).
RA, rheumatoid arthritis. IA, inflammatory arthritis. MΦ, macrophage. Mono, monocyte. Tex, exausted T cell. CTL, cytotoxic T cell.
REFERENCES: [1] Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 378 , 158-168 (2018).
[2] Calabrese LH, Calabrese C, Cappelli LC. Rheumatic immune-related adverse events from cancer immunotherapy. Nat Rev Rheumatol 14 , 569-579 (2018).
[3] Zhou X , et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: a systematic review and meta-analysis. Lancet Oncol 22 , 1265-1274 (2021).
[4] Braaten TJ , et al. Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis 79 , 332-338 (2020).
[5] Kostine M , et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann Rheum Dis 80 , 36-48 (2021).
[6] Thompson JA , et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network: JNCCN 20 , 387-405 (2022).
[7] Faje AT , et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 124 , 3706-3714 (2018).
[8] van Not OJ , et al. Association of Immune-Related Adverse Event Management With Survival in Patients With Advanced Melanoma. JAMA Oncol , (2022).
[9] Wu T , et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb ) 2 , 100141 (2021).
Acknowledgements: NIL.
Disclosure of Interests: None declared.