

Background: Subclinical gut inflammation, intestinal permeability and bacterial translocation are well described in inflammatory rheumatic diseases. While IL-17A is involved in the pathophysiology of both arthritis and inflammatory bowel diseases, the role of this cytokine in intestinal alterations related to rheumatic diseases has never been explored [1].
Objectives: Investigating the intestinal effects of an anti-IL-17A therapy (secukinumab) administered during the preclinical phase of adjuvant-induced arthritis (AIA) in rats, a model already known to present early intestinal alterations [2].
Methods: AIA was induced by injection of Mycobacterium butyricum in Freund’s incomplete adjuvant at the base of the tail in male Lewis rats (day 0). AIA rats were treated, or not, with secukinumab (20mg/kg/3days, i.p.) from day 0 to day 4 (pre-clinical phase) or to day 11 (arthritis onset). Body weights and arthritis scores were monitored daily. Intestinal inflammation was assessed by measuring mRNA expression of proinflammatory cytokines (RT-qPCR). Intestinal permeability and integrity were evaluated by the measure of plasma zonulin and iFABP levels (ELISA). TLR4 mobilization was estimated by serum sCD14 levels (ELISA).
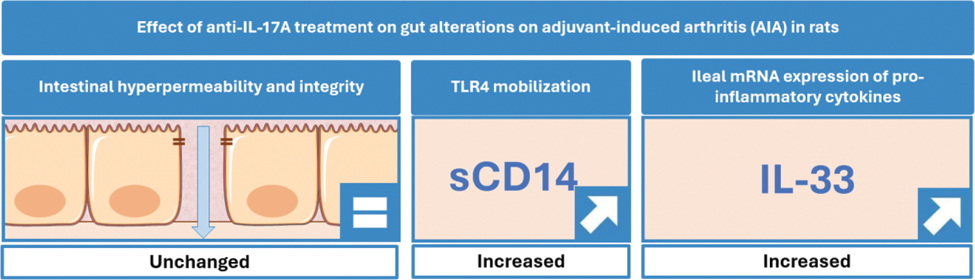
Results: As compared to untreated AIA, secukinumab did not change body weights and arthritis scores whatever the treatment duration. Plasma levels of zonulin and iFABP were unchanged by the treatment, both at day 4 and day 11. By contrast, higher serum levels of sCD14 were measured at days 4 and 11 under secukinumab treatment. Likewise, the treatment with secukinumab increased ileal mRNA expression of IL-33 but not IL-1β, IL-8, IL-17A, IL-23p19 and TNFα at days 4 and 11.
Conclusion: Blockade of IL-17A, during the preclinical phase of the AIA model, did not change intestinal permeability and epithelial damage but increased potential TLR4 mobilization and IL-33 gene expression. These data suggest a specific role of IL-17A in rheumatic-related intestinal changes and encourage further exploration of the IL-17A/IL-33 pathway in this setting.
REFERENCES: [1] Meyer F. et al. Autoimmun. Rev.2023;22(6):103331.
[2] Hecquet S. et al. Arthritis Res. Ther.2023;25(1):95.
Acknowledgements: For the financial support of French Society for Rheumatology (SFR).
Disclosure of Interests: None declared.