

Background: Apremilast is approved for the treatment of psoriasis and psoriatic arthritis (PsA). At the time of designing the REWARD study, real-world data regarding patients with PsA receiving apremilast in clinical practice in the Netherlands were lacking.
Objectives: Describe real-world use and effectiveness of apremilast for PsA in the Netherlands.
Methods: The observational REWARD study enrolled adults ≥ 18 years old initiating apremilast for PsA between November 2017 and March 2021. Patient-reported outcomes (PROs) included Health Assessment Questionnaire-Disability Index (HAQ-DI, 0 [no impairment] to 3 [maximum impairment]; response defined as total score ≤ 0.5 or a ≥ 0.3 decrease in the score from baseline [BL], at month 6 [M6], with nonresponder imputation) and PsA Impact of Disease (PsAID-12, 0 [best health status] to 10 [worst status]), both completed at BL, M3, M6 and M12; Short Form (36) Health Survey (SF-36, 0 [no disability] to 100 [maximum disability]), completed at BL and M12, and Treatment Satisfaction Questionnaire for Medication (TSQM, 0-100; higher scores=higher satisfaction) completed at BL, M6, M12. Clinical outcomes included tender and swollen joint counts (TJC-68 and SJC-66), enthesitis (Leeds Enthesitis Index [LEI] >0), dactylitis, adverse events (AEs) and adverse reactions (ADRs) (all assessed at BL, M3, M6 and M12, and analyzed as observed).
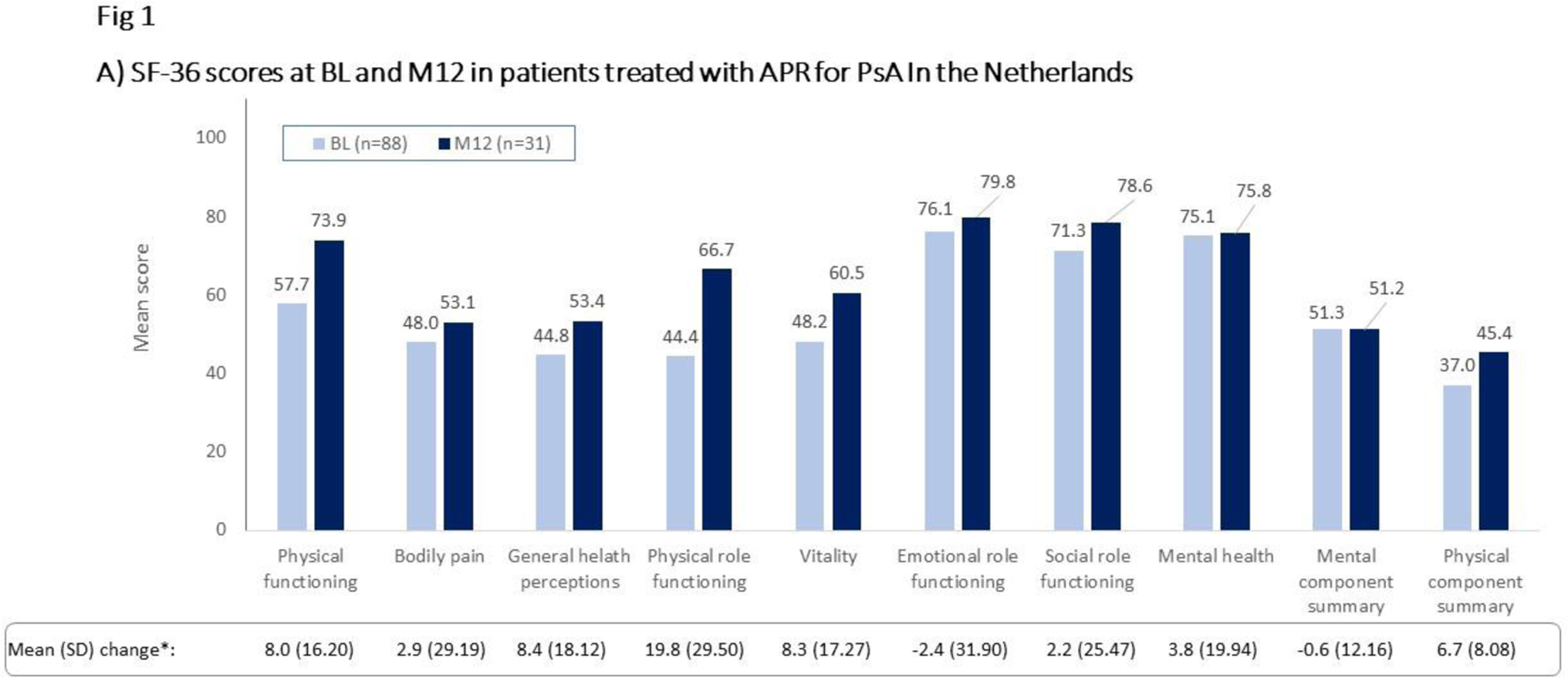
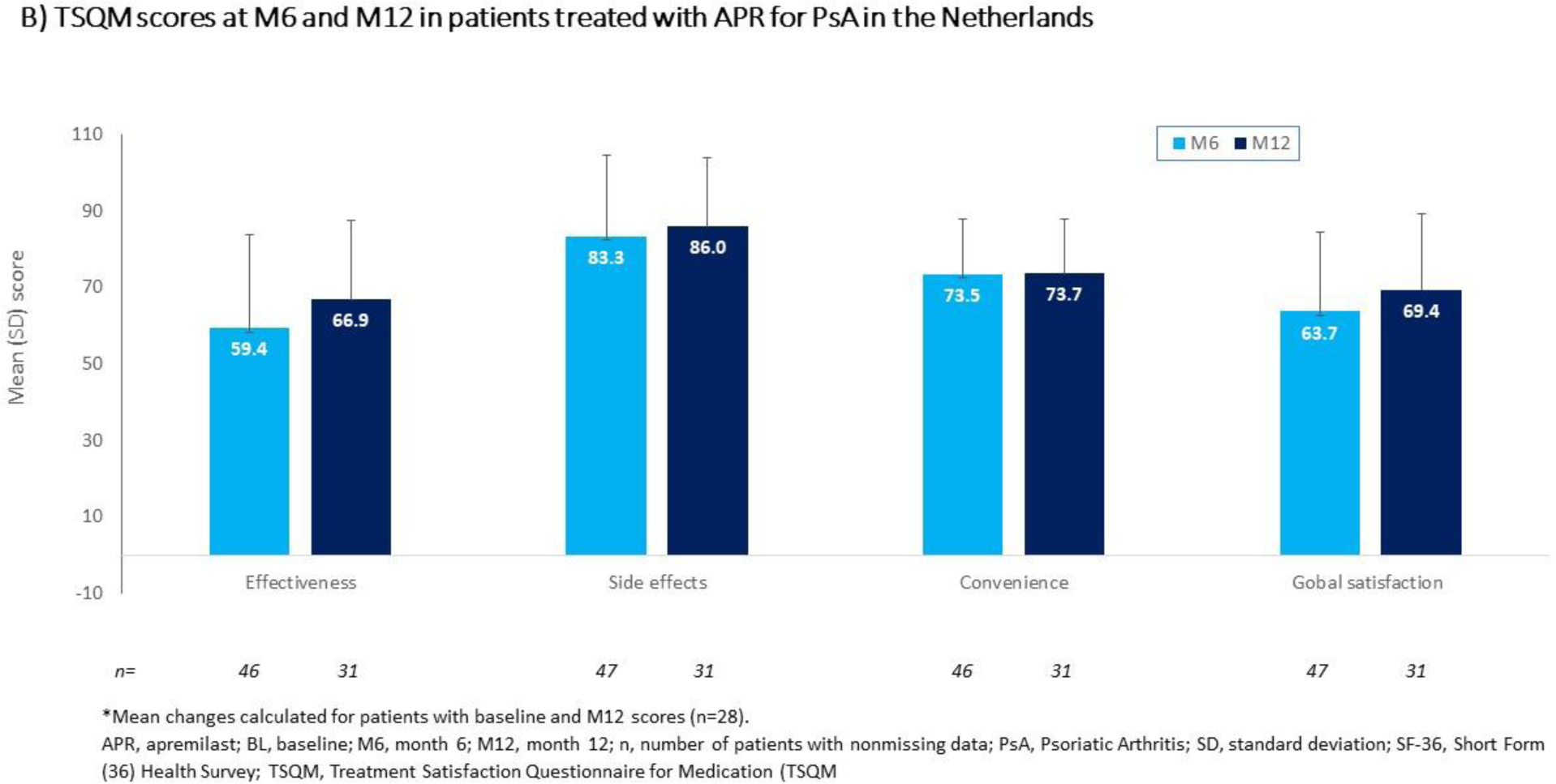
Results: Of the 99 patients enrolled, 49 (49.5%) were male and 50 (50.5%) were female, and the mean (SD) age was 53.1 (11.56) years. Mean (SD) duration of PsO and PsA was 14.6 (12.43) and 7.3 (7.95) years, respectively. Most patients (86/99 [87%]) had previously received disease-modifying antirheumatic drugs and initiated apremilast due to ineffectiveness of prior therapy (81/99 [82%]); approximately one-quarter (26/99) had previously received biologics. Mean (SD) BL HAQ-DI score was 0.78 (0.50) and one-third (33/99) of all patients achieved a HAQ-DI response at M6. The majority (55/99 [56%]) of patients completed M6 of apremilast treatment; 38 (38%) completed M12. Of these, over half achieved a HAQ-DI response: M6, 26/40 (65%); M12, 14/24 (58%) (data as observed). Mean (SD) PsAID score decreased from 4.54 (1.91) at BL (n=87) to 3.15 (1.92) at M6 (n=46) and to 2.89 (2.30) at M12 (n=29) (PsAID ≤4 considered clinically relevant). Most SF-36 scores increased on apremilast treatment (Figure 1A), with the largest improvements in Physical role functioning, General health perceptions, and Vitality. Mean (SD) TJC and SJC counts were 6.9 (9.26) and 3.7 (5.13) at BL (n=99) vs 3.0 (7.17) and 0.8 (2.47) at M12 (n=37), respectively. Enthesitis was seen in 40/99 (40%) of patients at BL vs 10/49 (20%) at M6 and 7/37 (19%) at M12. Mean (SD) dactylitis score was 4.4 (5.10) at BL (n=33) vs 0.5 (0.71) at M6 (n=18) and 1.0 (2.63) at M12 (n=14). At M6 and M12, mean (SD) TSQM scores were generally high (≥ 59), with patients reporting highest satisfaction with apremilast side effects (M6 [n=47], 83.3 [21.28]; M12 [n=31], 86.0 [18.06]) and convenience (M6 [n=46], 73.5 (14.25]; M12 [n=31], 73.7 [14.21]) (Figure 1B). The most commonly reported reasons for apremilast discontinuation were lack of effectiveness (30 of 99 patients [30%]) and AE (25/99 [25%]). The most commonly reported ADRs (>5% of patients) included diarrhea (20%), nausea (17%), and gastrointestinal disorder (6%). Serious ADRs were fatigue, decreased appetite, and depression (2% each).
Conclusion: In our real-world study of clinical practice, patients with PsA receiving apremilast for 6-12 months reported improvements in health and physical/emotional well-being, which was accompanied by reduced disease activity and high treatment satisfaction. No new safety signals were identified. While our data are limited by the sample size and high drop-out rate, the latter may reflect the range of treatments available in the Netherlands and suggest patients and physicians expect high levels of effectiveness, which can be achieved with apremilast treatment. In addition, our data highlight that PROs may be considered in treatment decisions.
REFERENCES: NIL.
Acknowledgements: Claire Desborough, Amgen Ltd, provided medical writing assistance.
Disclosure of Interests: Marijn Vis: None declared, Tim Jansen: None declared, Ian Bridges Amgen stockholder, Amgen employee, Margreet Plantenga Amgen stock holder, Amgen employee, Reinhard Bos: None declared.