

Background: Several composite outcome measures (COMs) are used for determining treatment effects in rheumatoid arthritis (RA), including the clinical disease activity index (CDAI), disease activity score for twenty-eight joints (DAS28), and the American College of Rheumatology 20%/50%/70% response (ACR20/50/70). Patient reported outcomes within COMs can be unreliable and may lack content validity. Patient reported and clinician reported outcomes may also capture different aspects of disease: patient reports may reflect quality of life, whereas clinician reports focus on clinical indicators. [1,2] While nuanced interpretations of COMs occur in a clinical setting, COMs historically have been interpreted as binary in the research context.
Objectives: To develop outcome measures that capture and quantify variability inherent in COMs which may enable improved molecular signature of response classifiers (MSRCs) when diseases incorporate qualitative endpoints to define response. COMS are typically binary indicators of patient status. We seek to provide a measure of response on a continuous scale via simulation to identify patients whose outcome measures are borderline due to test-retest variability; meanwhile also examining discordance among COMs assessed at the same time frame and quantify the magnitude of discordance. [3,4] An underlying hypothesis is that MSRCs trained using patients with discordant responses to therapy are less capable of discriminating drug response from nonresponse.
Methods: A cohort of 2449 patients from the TRIO Health database. Patients must have a diagnosis of RA; received at least one biologic with a CDAI > 10 within 45 days; and have ACR50, CDAI low disease activity/remission (CDAI-LDA/REM) and CDAI minimally important difference (CDAI-MID) data at 6 months after the index date, defined as first recorded date for biologic therapy. Monte Carlo simulated distributions of values for each component of ACR50, CDAI-LDA/REM, and CDAI-MID were created. Distributions were combined to create a measurement of response on a continuous scale from 0.0, reflecting nonresponse, to 1.0, reflecting response, with a score of 0.5 representing an ambiguous response. A total of 2000 simulated predictions were run for each patient. Patients with a score within a specified distance from 0.5 were removed from the set and concordance between ACR50, CDAI-LDA/REM, and CDAI-MID was evaluated. Jaccard similarity coefficient measuring concordance between COMs was performed for different distances from a score of 0.5. A Jaccard score of 0.0 reflects no concordance and a score of 1.0 reflects complete concordance.
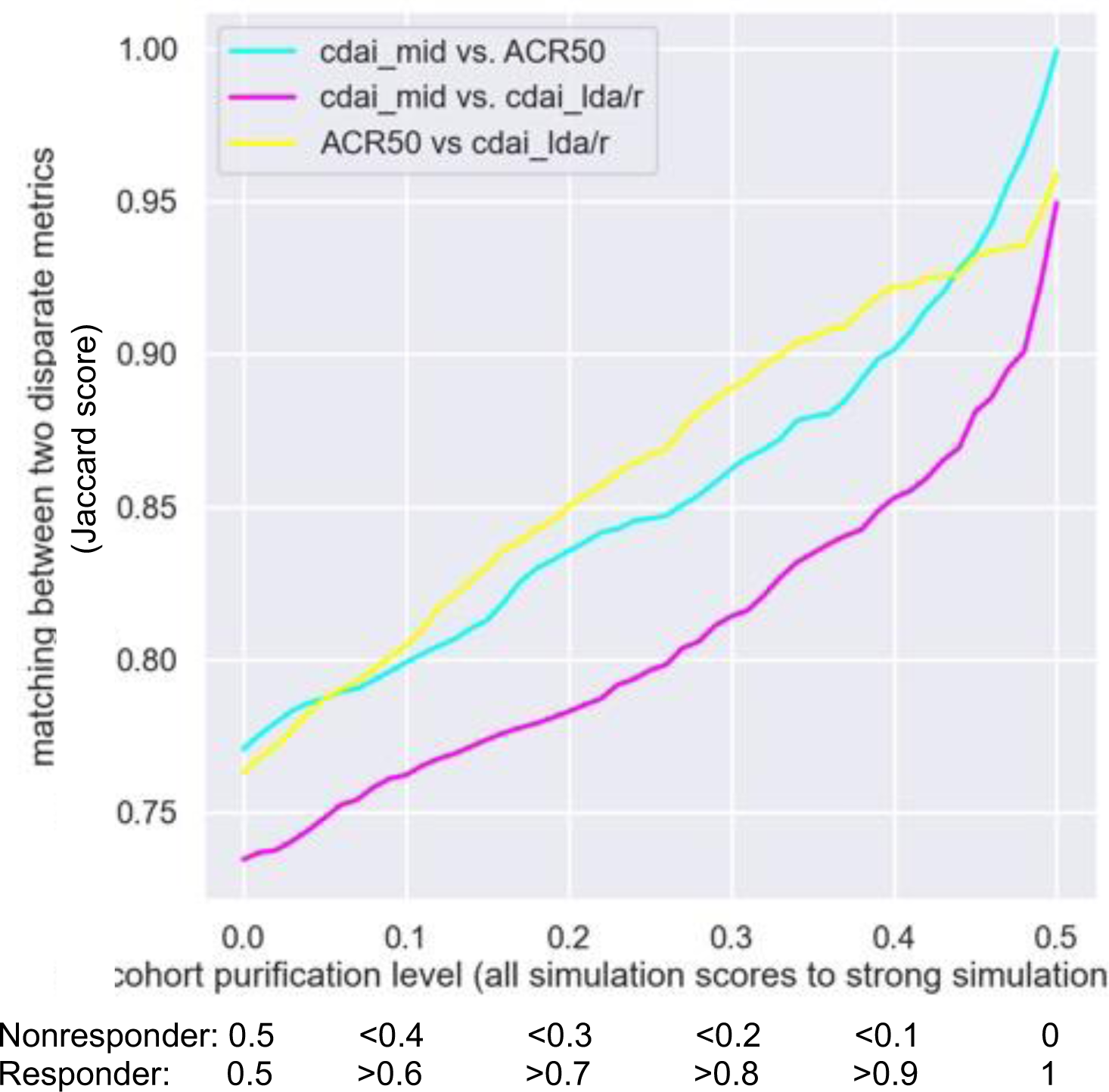
Results: Without removal of borderline patient responses, concordance between COMs was modest. Removal of patients within an increasing distance from a score of 0.5 resulted in significantly improved concordance. The CDAI-LDA/REM concordance with CDAI-MID improved from a Jaccard score of 0.73 at a distance of 0.0 to a score of 0.95 at a distance of 0.5 (see Figure 1). The concordance between ACR50 and CDAI-LDA/REM rose from 0.76 at a distance of 0.0 to a score of 0.96 at a distance of 0.5. The concordance of ACR50 to CDAI-MID increased from 0.77 at a distance of 0.0 to a score of 1.00 at a distance of 0.5.
Conclusion: The novel method described here qualifies the integrated variabilities introduced by individual clinical measurements and improves concordance between measures of therapy response used to develop MSRCs. MSRCs trained on data sets that remove borderline patient responses may better classify therapy responses and improve performance characteristics. By incorporating precision medicine tests with optimized performance characteristics, better informed medical management decisions could be made and requires further research.
REFERENCES: [1] Küçükdeveci, A. A. et al. RMD Open 7 , e001707 (2021).
[2] Fautrel, B. et al . Rheumatol Int 38 , 935–947 (2018).
[3] Smolen, J. et al. Arthritis Research & Therapy 21 , 231 (2019).
[4] American College of Rheumatology Committee to Reevaluate Improvement Criteria. Arthritis Rheum 57 , 193–202 (2007).
Jaccard concordance between different composite outcome measures.
Acknowledgements: NIL.
Disclosure of Interests: Nehad Soloman Glaxosmithkline, UCB, AbbVie Pharmaceuticals, Scipher Medicine, Labcorp, AstraZeneca, Janssen Pharmaceuticals, Amgen, Novartis, Lixia Zhang Scipher Medicine, Mark C. Zielinski Scipher Medicine, Sherry Guardiano Scipher Medicine, Jennifer Dines Scipher Medicine.