

Background: BHV-1300 is a first-in-class molecular degrader of extracellular protein (MoDE) that is being developed for individuals living with antibody-positive, moderate to severe rheumatoid arthritis (RA). Biological DMARDs, including tumor necrosis factor (TNF)-alpha inhibitors such as adalimumab, are one of the cornerstones of therapy in this difficult-to-treat population with significant unmet medical need.
BHV-1300 is a bifunctional molecule designed to target and then deliver pathogenic IgG autoantibodies, immune complexes to asialoglycoprotein receptors on hepatocytes for internalization and lysosomal degradation. MoDE IgG degraders such as BHV-1300 represent an entirely novel mechanistic class of agents for the treatment of IgG autoantibody-mediated diseases including RA, and importantly, MoDE IgG degraders offer the potential to be used in combination with biological DMARDs. Additionally, MoDE IgG degraders may also be able to reduce ADAs that have formed against biological DMARDs, restoring their earlier beneficial effects.
Objectives: The current study was undertaken to test the effect of BHV-1300 on adalimumab exposures in cynomolgus monkeys.
Methods: Six groups of 3 male naïve cynomolgus monkeys (N = 18) were administered adalimumab as a single 3 mg/kg subcutaneous injection at various times (−4 d, +2 h, +6 h, +12 h, +24 h, and +48 h) relative to a single 30 mg/kg intravenous bolus of BHV-1300. Plasma samples were collected at 24 hours post BHV-1300 administration. Serum samples were collected pre-dose (0) and at 2, 24, 48, 72, 96, 120, 144, 168, 336, and 504 hours post adalimumab administration. Concentrations of BHV-1300 in plasma samples were determined by liquid chromatography tandem mass spectrometry. Concentrations of adalimumab in serum samples were determined by enzyme-linked immunosorbent assay.
Results: Adalimumab and BHV-1300 pharmacokinetics results are provided in Table 1. Exposure (maximum concentration and area under the curve) to adalimumab was lowest when the drug was administered 2 hours after BHV-1300 and relatively similar at all other dosing times. Adalimumab PK parameters (i.e., Cmax, Tmax, and AUC 0-168 ) between 6 and 48 hours post-dosing with BHV-1300 were all within ±20% of historical values. Plasma concentrations of BHV-1300 at 24 hours post administration showed less than 1.5-fold differences across the 6 groups.
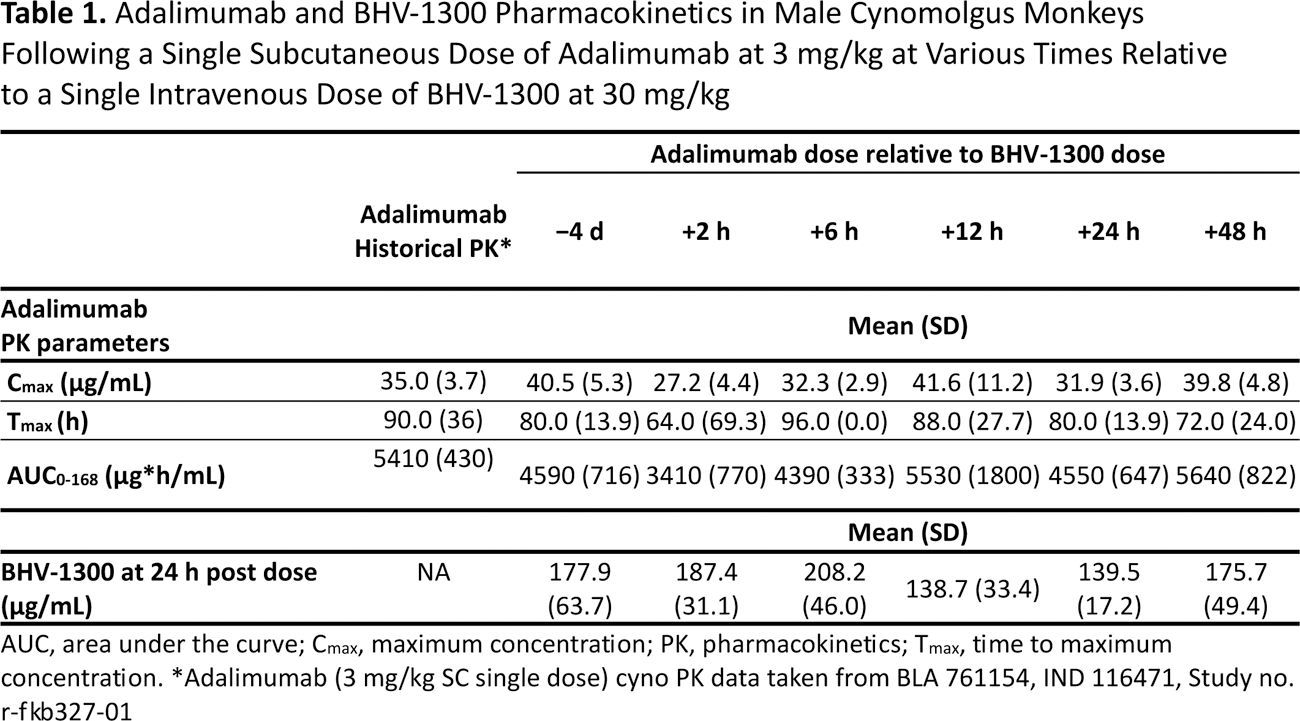
Conclusion: These data demonstrate that BHV-1300 does not adversely affect exposure to adalimumab and provide supporting evidence that MoDE IgG degraders such as BHV-1300 can be effectively used in combination with biological DMARDs in the management of difficult-to-treat autoimmune diseases. These results represent a key differentiating feature from neonatal Fc receptor inhibitors, which adversely affect the exposure to and effectiveness of, and cannot be co-administered with, Fc-containing biologics, including adalimumab. Moreover, BHV-1300 potentially offers a significantly improved benefit-risk based on other important mechanistic advantages including rapid onset of IgG-lowering, shortened time to maximal effect, depth of IgG lowering, brief period of exposure, potential for reduced immunosuppression, and lack of effects on albumin, cholesterol or triglycerides.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: Elizabeth Dierks As part of my investment portfolio and as partial compensation for my jobs, I have a small amount of stock in Biohaven Pharmaceuticals and Bristol Myers Squibb, of which both companies have programs for drugs to treat rheumatology., I am currently an employee of Biohaven Pharmaceuticals. I have previously worked at Bristol Myers Squibb., Peter Ackerman I am a Biohaven employee and shareholder., I am a Biohaven employee and shareholder., Dennis Heller I am currently a consultant via Certara (my employer)., Anna Bunin Biohaven Pharmaceuticals, Frank Engler I am a Clinical Pharmacology Consultant and have been working with Biohaven for 2+ years., Brian Gavin I am a shareholder of Bristol Myers Squibb., I am a former employee of Bristol Myers Squibb., I have consulted for Soley Therapeutics and Biohaven Pharmaceuticals., David Pirman I own shares in Biohaven., I have been employed by Agios and Biohaven., I worked previously for a startup pharma company., Bruce Car We are working on novel degraders for rheumatologic indications at Biohaven of which I am a shareholder., I work for Biohaven., Irfan Qureshi I am an employee and shareholder of Biohaven., Vlad Coric I am an employee and own stock in Biohaven (NYSE: BHVN)., I am an employee and own stock in Biohaven (NYSE: BHVN)., I am an employee and own stock in Biohaven (NYSE: BHVN)., I am an employee and own stock in Biohaven (NYSE: BHVN).