

Background: Terminally differentiated T effector memory re-expressing CD45RA (Temra) are enriched in rheumatoid arthritis (RA) 1 . Temra have cytotoxic and pro-inflammatory properties but their roles in RA pathophysiology and disease activity are unclear. CD4 Temra can be generated through exposure to viruses, with cytomegalovirus (CMV) being a major culprit 2 .
Objectives: To investigate the role of CD4 Temra in RA.
Methods: A cross-sectional cohort of patients with RA was recruited from rheumatology clinics. Multi-parameter flow cytometry was performed on peripheral blood mononuclear cells. FlowJo version 10.8 was used to analyse live-gated lymphocytes. CMV IgG positivity was determined by ELISA. Mann-Whitney test or unpaired t-test were applied as appropriate, analysis of variance (ANOVA) of log-transformed values was used to adjust for age. Chi-squared test or Fisher’s exact test were used to compare categorical data. Spearman rank correlation was used to compare correlation between Temra and CMV IgG titre.
10X single cell RNA sequencing was performed on CD4 T cells sorted by fluorescence-activated cell sorting (FACS) and analysed with the Seurat package using Rstudio version 4.1.2. Ingenuity Pathway Analysis was used for further analysis.
Cytotoxicity assays were performed by incubating FACS-sorted T cell subsets with K562 cells at a ratio of 20:1 in the presence of IL-15 for 4 hours. After centrifugation, supernatants were transferred to a black flat-bottomed plate and fluorescence intensity analysed using a spectrophotometer. % Cytotoxicity was calculated using:
100 × (Sample fluorescence intensity - Spontaneous lysis fluorescence intensity)Maximum lysis fluorescence intensity -Spontaneous lysis fluorescence intensity
Wilcoxon matched-pairs signed rank test was used to compare CD4 Naïve and CD4 Temra in cytotoxicity assays.
Results: 36 patients with RA treated with anti-TNF and 14 healthy individuals were recruited. Increased serum IgG CMV positivity (93% vs 42%, p = 0.009), and increased frequency and numbers of peripheral CD4 Temra (p = 0.004 and p = 0.006 respectively) were observed in patients with active RA treated with anti-TNF compared to those in remission. Serum CMV IgG titre correlated with the frequency of CD4 Temra of patients with RA on anti-TNF (Spearman r = 0.38, p = 0.03) but not with patient age.
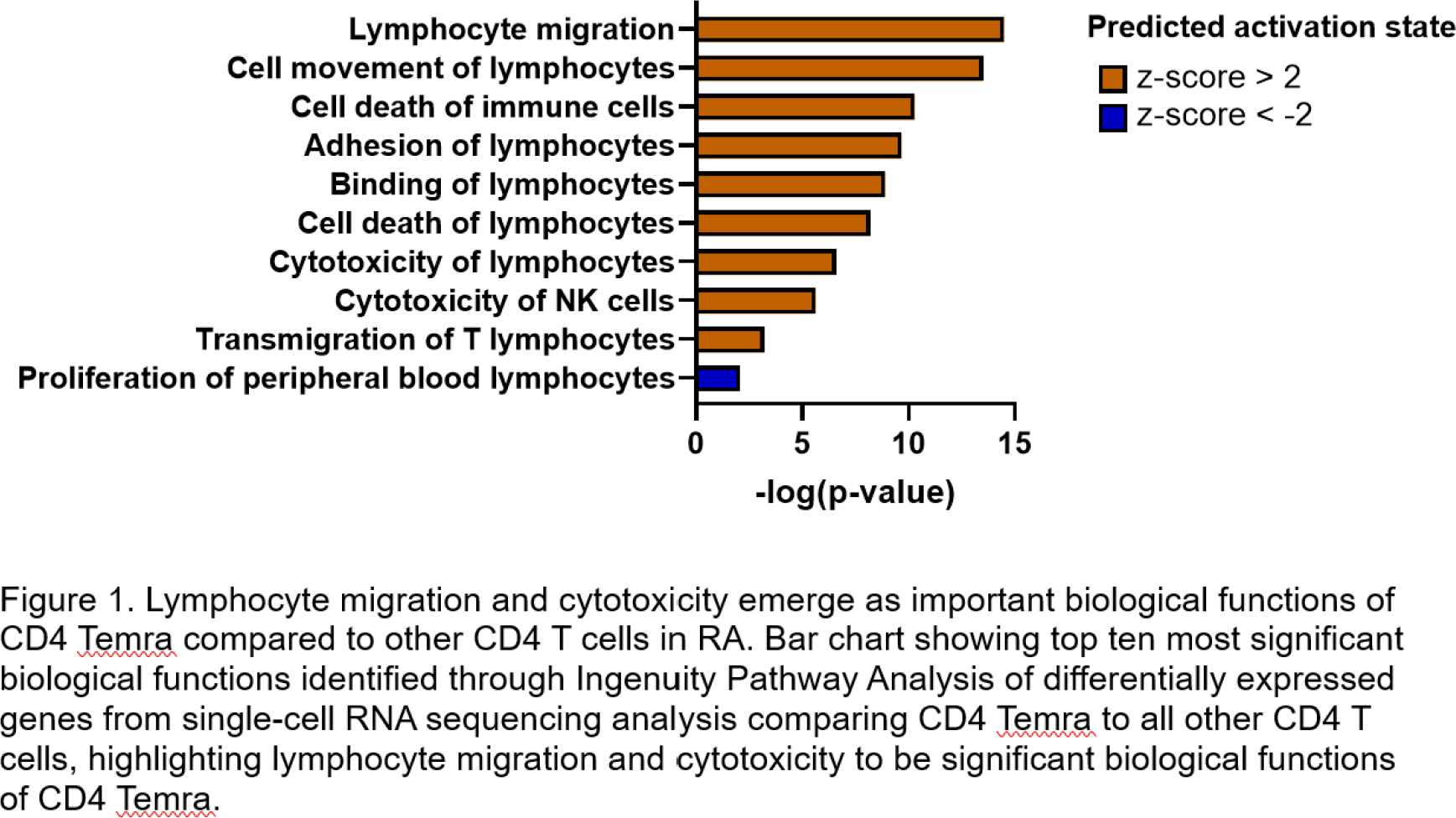
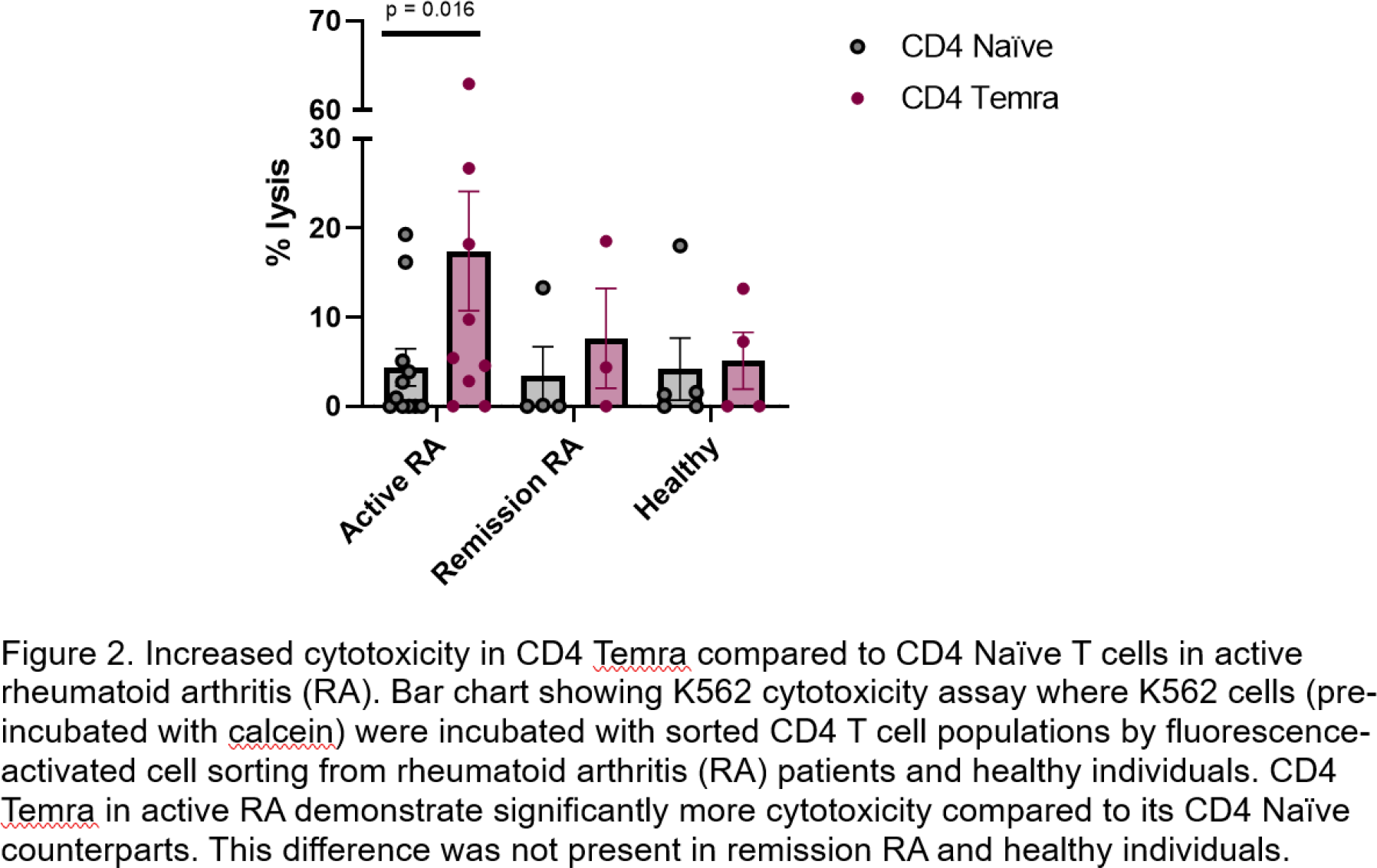
10X single cell RNA sequencing was performed on FACS-sorted CD4 T cells (14 samples comprising 6 patients with RA before and after anti-TNF therapy and 2 healthy controls). 10 clusters were identified, including a CD4 Temra cluster. Terminal differentiation was confirmed using pseudotime lineage analysis, with CD4 T effector memory cluster being the immediate precursor. The CD4 Temra cluster expressed an increased lymphocyte adhesion and migration signature, and cytotoxicity compared to other clusters (Figure 1). A functional cytotoxicity assay showed that CD4 Temra demonstrated increased major histocompatibility complex (MHC) independent cytotoxicity compared to CD4 Naïve T cells in patients with active RA (p = 0.016) but this difference was not seen in those in remission or healthy individuals (Figure 2).
Conclusion: The enhanced CD4 Temra frequency and cytotoxicity in patients with active RA suggest a role in perpetuating disease. Single cell RNA sequencing analysis of CD4 Temra reveal their propensity to migrate towards inflammatory sites as well as cytotoxicity compared to other CD4 cell populations. The association between CMV IgG titre, Temra frequency and disease activity provide a plausible mechanism by which CMV may aggravate inflammation in RA.
REFERENCES: [1] Weyand CM, Yang Z, Goronzy JJ. T-cell aging in rheumatoid arthritis. Curr Opin Rheumatol. 2014;26(1):93-100.
[2] Libri V, Azevedo RI, Jackson SE, et al. Cytomegalovirus infection induces the accumulation of short-lived, multifunctional CD4 + CD45RA + CD27 − T cells: the potential involvement of interleukin-7 in this process. Immunology. 2011;132: 326-339.
Acknowledgements: The authors would like to acknowledge Versus Arthritis, Rosetrees Trust, Royal College of Physicians, University College London Hospitals Biomedical Research Centre, University College London Hospitals Charity for funding this research, as well as patient partners and University College London Hospital Patient Partners in Rheumatology Research for their input.
Disclosure of Interests: Su-Ann Yeoh: None declared, Michael Ehrenstein: None declared, Arne Akbar: None declared, Daniel McCluskey: None declared, Luisa Chocarro: None declared, Wing Tung Ma: None declared, Daniel Janman Current employee of Quell Therapeutics (involved in modular engineered Treg, unrelated to the current research). Involvement in the submitted research was whilst affiliated with University College London, Jamie Evans: None declared, Eleanor Hawkins: None declared, Linda Tomson: None declared.