

Background: Axial spondyloarthritis (axSpA) is a chronic autoinflammatory disease that primarily affects the axial skeleton. Association studies support not only a genetic contribution in disease development, but also a strong involvement of both immunologic and environmental factors. Expression studies show the importance of inflammatory and immunomodulating activity in the onset and development of the disease. In this context, peroxisome proliferator-activated receptor (PPAR)-γ has previously been found involved in ankylosing spondylitis [1] and is known to be an inhibitor of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and its related inflammatory [2].
Objectives: To characterize antioxidant and inflammatory response in AxSpa patients through the analysis of peripheral blood mononuclear cells (PBMCs), providing a window into the pathogenesis of numerous diseases [3]. The subsequent goal was to correlate the molecular alterations with the different grades of disease measured through the Ankylosing Spondylitis Disease Activity Score (ASDAS).
Methods: Quantitative real time PCR (qRT-PCR) was employed to measure PPAR genes (α, γ, and δ) in PBMCs obtained from both healthy and axSpA subjects. Antioxidant and inflammatory response was assessed by RNAsequencing. Functional enrichment analysis was performed by using g:Profiler. Pearson’s correlation analyses were performed using Matlab R2021b (Version: 9.11.0.2207237, The MathWorks, Inc.).
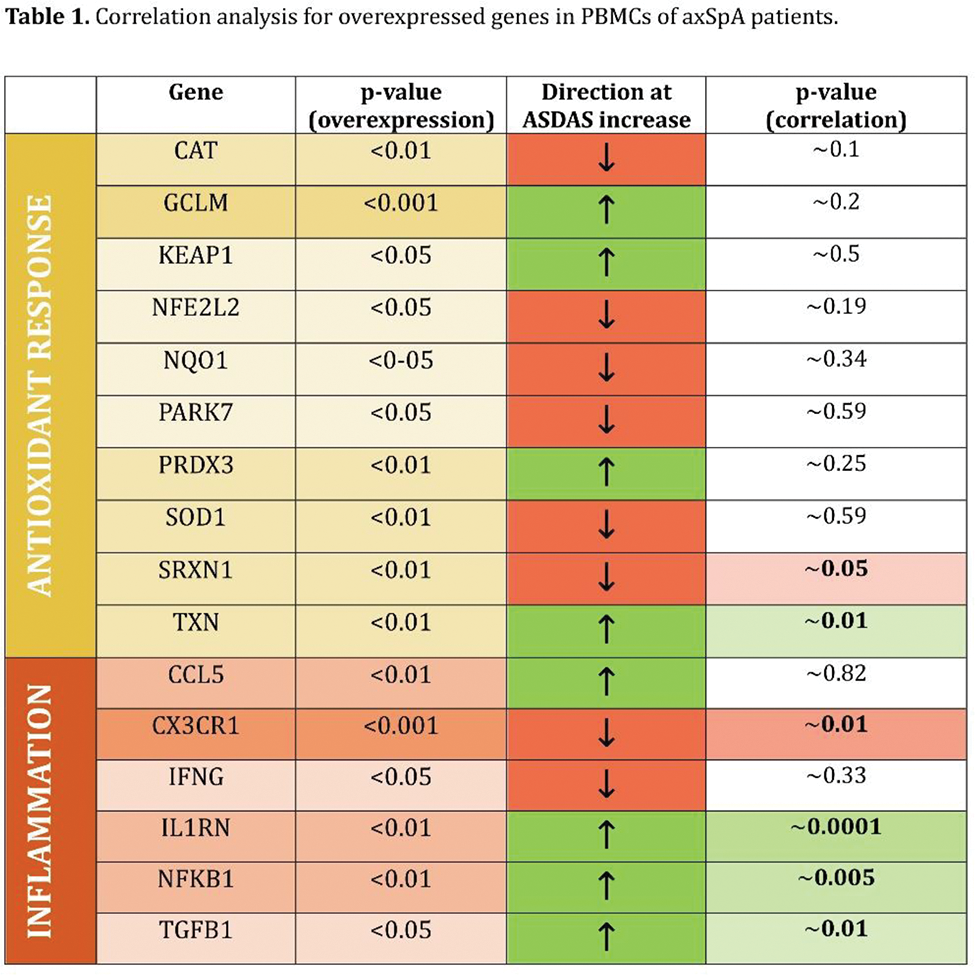
Results: Among PPAR genes, PPAR-γ was found to be significantly down-regulated in AxSpA patients compared to healthy subjects. According to this result, 16 genes related to oxidative stress response (e.g., NFE2L2 , NQO1, SOD1, and CTA ) and/or inflammation (e.g., NFKB1 , IFNG , IL1RN , TGFB1 ) showed to be over-expressed ( Table 1 ). Association of gene expression values to ASDAS grading score demonstrated a negative Pearson’s correlation index for markers related to oxidative stress response, while a positive correlation intercurred between genes related to inflammation/immunoregulation and disease severity. In addition to that, PPAR-γ expression increased with the severity of the disease. Functional enrichment analysis showed the involvement of antioxidant activity, cytokine receptor binding, cellular response to chemical stimuli, and regulation of molecular function clusters in the disease.
Conclusion: Together, these findings support the evidence for a role of PPAR-γ deficiency, NF-κB signalling pathway activation, and Nrf2-related antioxidant response in AxSpA progression.
* Giuseppe Caruso and Florenzo Iannone equally contributed as last author.
REFERENCES: [1] Lin, A., et al., Lipocalin 2 links inflammation and ankylosis in the clinical overlap of inflammatory bowel disease (IBD) and ankylosing spondylitis (AS). Arthritis Res Ther, 2020. 22 (1): p. 51.
[2] Ju, Z., et al., Anti-inflammatory effects of an optimized PPAR-gamma agonist via NF-kappaB pathway inhibition. Bioorg Chem, 2020. 96 : p. 103611.
[3] Phongpreecha, T., et al., Single-cell peripheral immunoprofiling of Alzheimer’s and Parkinson’s diseases. Sci Adv, 2020. 6 (48).
Acknowledgements: NIL.
Disclosure of Interests: None declared.