

Background: Axial Spondyloarthritis (axSpA) is a disease continuum ranging from non-radiographic disease in the lumbar spine and sacroiliac joint to progressive radiographic changes known as Ankylosing Spondylitis (AS). Approximately 60% of individuals with Ankylosing Spondylitis experience mucosal inflammation and flares of mucosal inflammation are associated with joint inflammation (Mielants et al., 1995). Although dysbiosis is present in Ankylosing Spondylitis (Ciccia et al., 2017), its role in disease development remains unknown. Dendritic cells (DCs) are specialized immune cells equipped with receptors for microbes and cell damage. They guide the selection and proliferation of appropriate effector cells. It is vital then, that immune cells must adapt to variable environments, like the hypoxic mucosal surface of the gut, hence many immune signalling pathways overlap with oxygen-sensing mechanisms (Taylor and Colgan 2017). Notably, hypoxia is intrinsic to the gut mucosa, joints, and inflammation—key sites associated with Ankylosing Spondylitis, hence immune responses in this context should be explored.
Objectives: This study investigated the role of gut bacteria and hypoxic cell stress in inflammatory immune responses in axSpA.
Methods: DCs were derived from axSpA and Healthy Control blood monocyte cells, then stimulated with heat-killed Bacteroides fragilis (a commensal isolated from healthy control in a previous study (Stebbings, Taylor et al. 2009)) or Campylobacter jejuni (a pathogen) under normoxic (atmospheric oxygen) or hypoxic (1% oxygen) conditions. DC activation was measured using flow cytometry for surface markers and cytokine secretion was assessed by Bioplex (n=10, age and sex-matched controls). The effect of DC activation on T cell responses was assessed through coculture experiments followed by intracellular cytokine staining and analysis by flow cytometry. Patient results were stratified based on Ankylosing Spondylitis Disease Activity Scores (ASDAS): low disease activity (Low ASDAS < 2.1) and high disease activity (High ASDAS ≥ 2.1).
Results: Patients exhibited variable activation marker expression, but MHC-I and IL-1β were significantly higher in the Ankylosing Spondylitis (AS) group when stimulated with both C. jejuni and Bacteroides under normoxic conditions. Hypoxic conditions reduced differences, with only MHC-I remaining elevated during Bacteroides stimulation (p=0.0047). When stratified by disease activity, DCs from Low ASDAS patients showed heightened responsiveness to bacterial stimuli, including the Bacteroides isolate CAS10, while Healthy Controls only upregulated the suppressive cytokine, IL-10. In contrast, the High ASDAS group displayed weaker DC responses to stimulation; in hypoxic conditions only MHC-I remained significantly elevated. T cell coculture experiments indicated significantly increased IL-17+ and IL-10+ CD4+ T cell responses.
Summary of significant AS MoDC responses compared to Healthy Controls in normoxia and hypoxia. Statistical analysis by Mann Whitney test ‘*’ p<0.05, ‘**’ p< 0.01, ‘***’ p< 0.001. Ankylosing Spondylitis Disease Activity Score ASDAS; CAS10, Bacteroides fragilis isolate CAS10
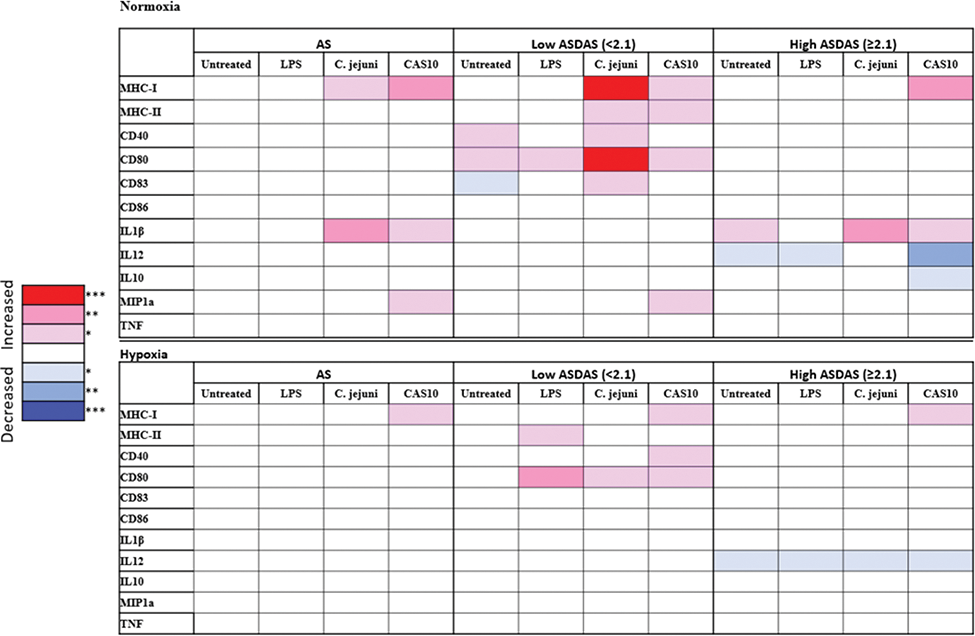
Conclusion: Based on these findings, we hypothesize that heightened DC responses to commensal bacteria in axSpA lead to abnormal T cell priming in hypoxic conditions, such as the gut or joints.
REFERENCES: [1] Ciccia, F., et al. (2017). “Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis.” Annals of the rheumatic diseases 76 (6): 1123-1132.
[2] Mielants, H., et al. (1995). “The Evolution of Spondyloarthropathies in Relation to Gut Histology. II. Histological aspects.” The Journal of Rheumatology 22 (12): 2273-2278.
[3] Stebbings, S. M., et al. (2009). “The immune response to autologous bacteroides in ankylosing spondylitis is characterized by reduced interleukin 10 production.” The Journal of Rheumatology 36 (4): 797-800.
[4] Taylor, C. T. and S. P. Colgan (2017). “Regulation of immunity and inflammation by hypoxia in immunological niches.” Nature Reviews Immunology 17 (12): 774-785.
Acknowledgements: NIL.
Disclosure of Interests: None declared.