

Background: Individuals diagnosed with systemic lupus erythematosus (SLE) experience a heightened and earlier onset of cardiovascular (CV) events when compared to healthy individuals [1, 2]. However, data on appropriate CV risk assessment tools and accurate surrogate markers are scarce [3].
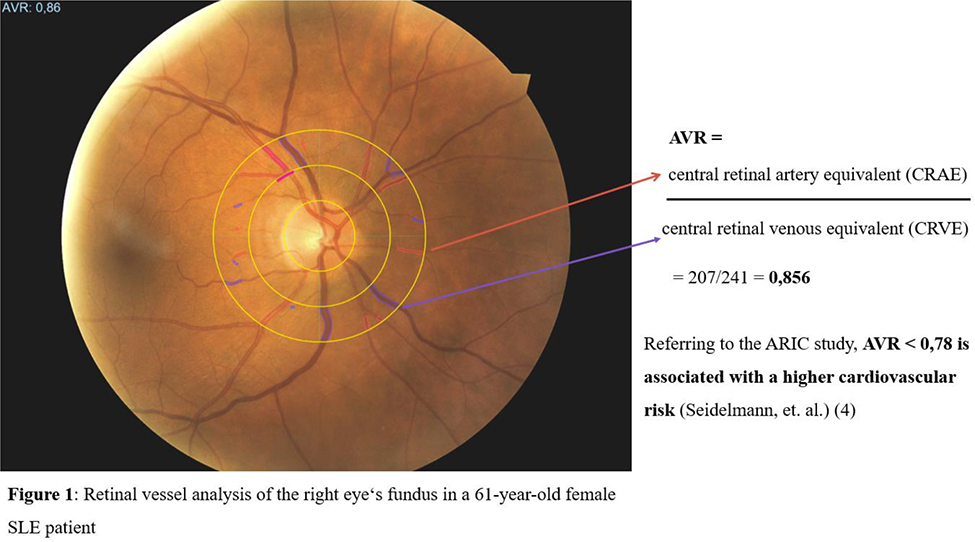
Objectives: The study aimed to investigate novel CV and cerebrovascular (CVB) markers like retinal vessel analysis (RVA) (Figure 1) and ophthalmic artery Doppler, next to well-established surrogate parameters of angiopathy and CV-risk (aortic stiffness and carotid ultrasonography) in a large SLE cohort [Systemic LUPus erythematosus associated CARDiovascular risk; LUPCARD]. Moreover, to evaluate correlations of these markers with disease- and patient-associated characteristics, as well as with traditional CV risk factors.
Methods: The LUPCARD study focuses on CV/CVB-risk examination in patients treated for SLE (EULAR/ACR-criteria) in a multicenter study setting. Patients and healthy controls underwent ultrasound and oscillometric examinations, including aortic stiffness assessment (carotid-femoral pulse wave velocity; cfPWV), Doppler- and B-mode sonography of common (CCA) and internal carotid arteries (ICA) for plaque evaluation, carotid intima-media thickness (cIMT), as well as resistance- and pulsatility-indices (RI, PI). In addition, Doppler examinations of the ophthalmic arteries and retinal vessel analysis (RVA) via a static RVA model were performed to determine the ophthalmic arteriovenous-ratio (AVR) and the central retinal arterial/venous equivalents (CRAE, CRVE).
Results: 185 SLE patients [89.2%♀, 53 years (41-59, IQR)] and 88 healthy control subjects [84.1%♀, 51 years (37-58, IQR)] were recruited. CCA-RI [0.75 (0.7-0.8, IQR) vs. 0.72 (0.69-0-76, IQR); p=0.016] and ICA-RI [0.67 (0.61-0.73, IQR) vs. 0,61 (0.09, SD); p<0.001], as well as CCA-PI [1.65 (1.44-2, IQR) vs. 1.58 (0.44, SD); p=0.029] and ICA-PI [1,23 (1.03-1.53) vs. 0.96 (0.85-1.28); p<0.001] were significantly higher in the patient group, respectively. Furthermore, SLE patients showed significantly more carotid plaques [45.5%, vs. 14.8%; p=0.026] and higher cfPWV-values [which however did not reach significance: 7.1 m/s (6.1-8.5, IQR) vs. 6.9 m/s (1.3, SD); p>0.05].
Regarding associations among patients, CCA-RI correlated with C-reactive protein (p=0.05) and was higher in SLE patients with restrictive lung function profiles (VC in : rho= -0.346, p=0.016).
cfPWV associated mainly with traditional CV risk factors like age (rho=0.528; p<0.001), mean arterial pressure (rho=0.381; p<0.001), and hyperlipidemia (rho=0.329; p<0.001), as well as (inversely) with lung CO-diffusion (p=0.003).
Interestingly, AVR correlated significantly with the pulsatility/resistance indices of the CCA [p=0.009; rho=-0.406]/[p=0.001; rho=-0.489], ICA [p=0.008; rho=-0.416]/[p=0.016; -0.378] and with the PI of the ophthalmic artery (p=0.048).
Conclusion: In one of the largest surrogate marker cohorts in SLE, patients had significantly higher carotid resistance and pulsatility, as well as plaque formation than controls. Key predictors of macro- and microvascular damage could be identified. Moreover, retinal vessel analysis and ophthalmic artery Doppler revealed signs of subclinical microangiopathy, which could assist identification of CV disease in an early stage. Thus, the evaluated surrogate markers may be of value during CV/CVB screening and improve overall SLE prognosis.
REFERENCES: [1] Tsokos GC. New England Journal of Medicine. 2011. 365(22): 2110-2121.
[2] Stortz M, et al., Clin Exp Rheumatol. 2020;38(1): 74-81.
[3] Triantafyllias K, et al. Rheumatology (Oxford). 2021;60(3):1300-1312.
[4] Seidelmann SB, et al. Circulation. 2016;134(18):1328-1338.
Acknowledgements: NIL.
Disclosure of Interests: Konstantinos Triantafyllias Pfizer, Novartis, Galapagos, UCB, Janssen, Chugai, Sanofi, AbbVie, Pfizer, Galapagos, Felix Tietze: None declared, Andreas Schwarting: None declared.