

Background: Recent evidences support the usefulness of belimumab (BEL) on ameliorating general disease activity and acute lupus nephritis (LN), while reducing the dose of prednisolone (PSL) in systemic lupus erythematosus (SLE). BEL is administrated either intravenously or subcutaneously, and little information exists regarding to the satisfaction and outcome of patients using subcutaneous BEL and its effect on chronic phase of LN.
Objectives: This study aimed to investigate the effects of subcutaneous BEL on PSL dose reduction, disease activity, and patient satisfaction using real-world data in long-standing patients with SLE.
Methods: A total of 106 patients with SLE who received subcutaneous BEL were included, and 76 patients who started BEL treatment at least one year prior were evaluated. Baseline characteristics of former BEL (N = 16) and current (N = 60) users were compared. Then, the former BEL users were excluded, and the current users were analysed. To clarify the efficacy induced by BEL itself, we adopted three time points (around one-year before BEL induction: “Year -1”, BEL induction point: “Year 0”, and around one-year after BEL induction: “Year 1”), and compared the changes from “Year -1” to “Year 0” and those from “Year 0” to “Year 1”. Clinical information, including retention rate, disease activity, and renal outcome was evaluated. Patient satisfaction was further evaluated using Treatment Satisfaction Questionnaire for Medication (TSQM-II).
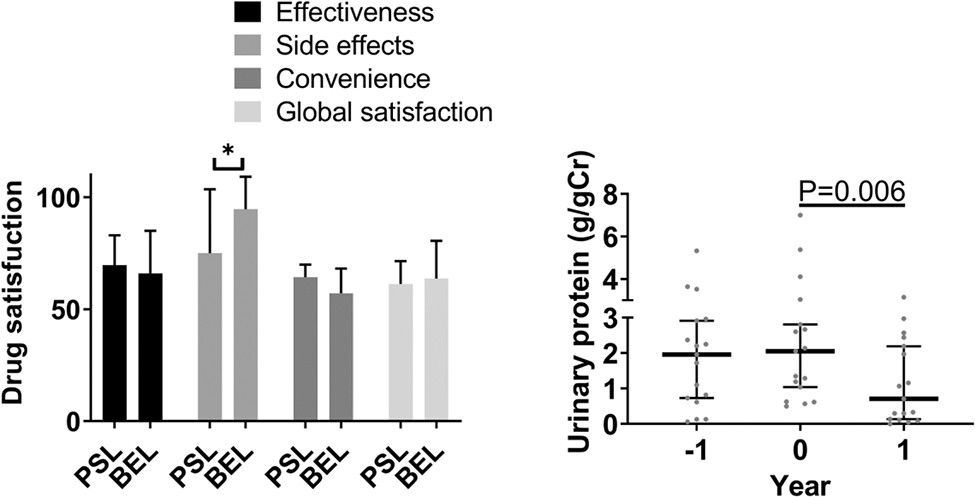
Results: The retention rate of subcutaneous BEL was high and accounted for >80% after two years. Ineffectiveness and pain were the major reasons for discontinuation of BEL treatment. A satisfaction survey comparing subcutaneous BEL and PSL using the TSQM revealed significantly higher satisfaction with the side effects of BEL. However, the convenience was less satisfied with the subcutaneous BEL. Effectiveness and global satisfaction were comparable. BEL significantly improved SLEDAI-2K scores, LN, and PSL dosage, with a median reduction of 4 mg/day. The reduction in SLEDAI-2K levels was attributed to improvements in arthritis, proteinuria, and serological parameters, including low complement and anti-double-stranded DNA antibodies. The effect of BEL on lowering proteinuria was significant regardless the change in the dose of PSL. Even in class V, urinary protein was reduced in 66.7 % of patients. The contributing factors to reduce PSL were active disease and positive C1q-binding immune complex, and PSL reduction ≥5 mg was achievable in such cases. Patients with PSL reduction of ≥5 mg showed significantly lower blood low-density lipoprotein and triglyceride by 13 and 17 mg/dL, respectively, while those with PSL reduction of <5 mg remained unaltered.
Conclusion: The retention rate of subcutaneous BEL was high although trouble with injection was the main reason leading to the discontinuation. Patient satisfaction with adverse effects was higher with subcutaneous BEL than that with PSL. BEL effectively lowered the PSL dosage and improved disease activity and LN, even in long-lasting patients. PSL reduction was achievable in active disease and positive C1q-binding immune complex, and metabolic parameters were also improved in such cases. Inject-site pain was the major complaint of subcutaneous BEL, and its improvement will increase the retention rate and patient satisfaction.
REFERENCES: NIL.
Acknowledgements: NIL.
Disclosure of Interests: None declared.