

Background: Rebamipide is a widely used gastroprotective agent. Beyond its muco-protective effects, this agent presented immune-modulatory effects for various rheumatologic diseases such as rheumatoid arthritis, osteoarthritis, Sjogren’s syndrome, and Behcet’s disease in multiple pre-clinical and clinical studies. However, its efficacies on lupus have not been evaluated yet.
Objectives: Here, we determined the therapeutic potentials of rebamipide in lupus animal models and suggested possible mechanisms.
Methods: We administered rebamipide (5 mg/kg) or vehicles in 8-week-old MRL/lpr mice by daily oral feeding for 8 weeks and determined therapeutic efficacies on lupus-like phenotypes and immune cell profiles using their sera and tissues. Through in vitro experiments using immune cells and podocytes acquired from mice, and podocyte cell lines, potential therapeutic mechanisms were evaluated.
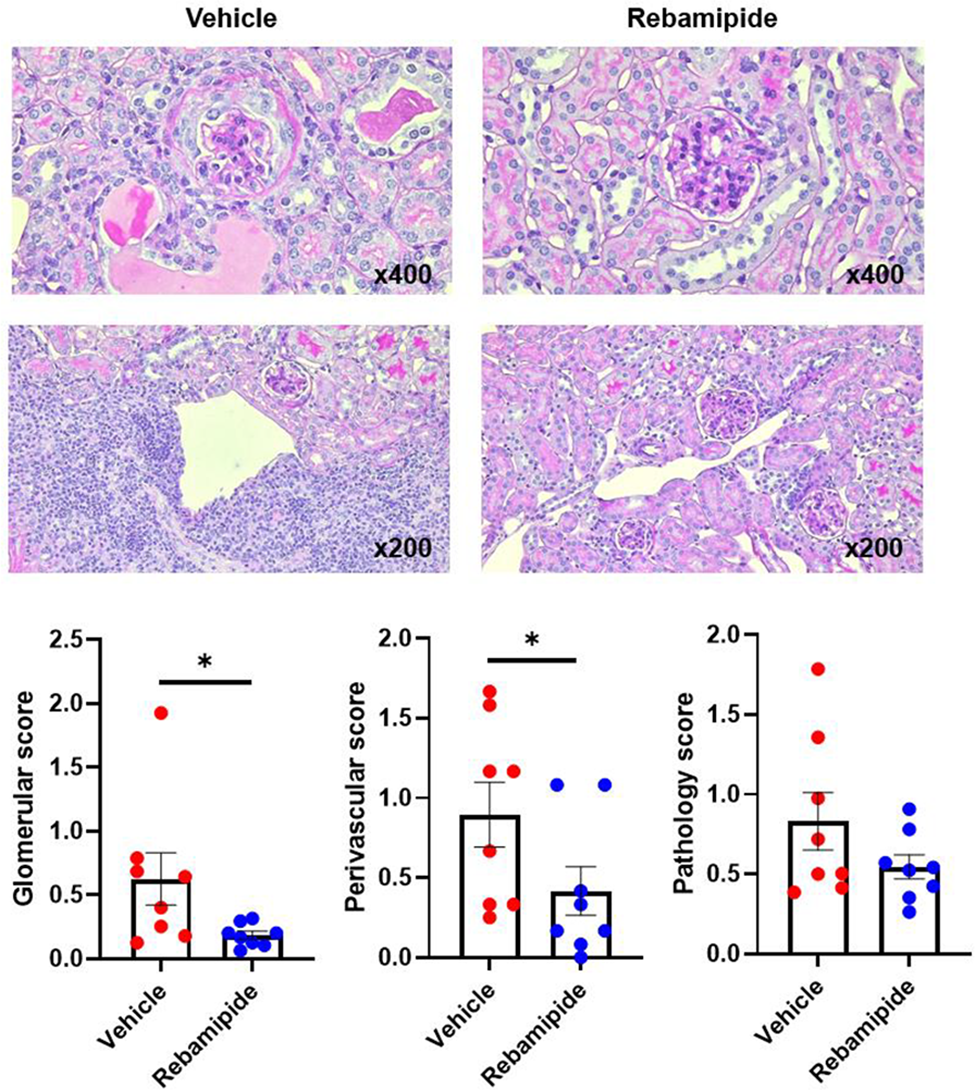
Results: Oral rebamipide administration in lupus-prone mice significantly reduced proteinuria and pathologic scores in renal tissues with increased expression of podocyte-related proteins such as nephrin, synaptopodin, and podocin. Sizes of spleen tissues were significantly more decreased in mice treated with rebamipide than those treated with vehicles. Rebamipide increased the proportion of circulating regulatory T cells in lupus-like mice. In contrast, other immune cell populations such as double negative T cells, CD4+ T cells, CD8+ T cells, and plasma cells were not changed by in vivo treatment of rebamipide. In vitro treatment of rebamipide in primary-cultured murine podocytes resulted in increased expression of structural proteins of podocytes including synaptopodin and podocin, as well as heme oxygenase-1, which is one of the anti-oxidant proteins. These results were replicated in experiments using human and murine podocyte cell lines.
Conclusion: Rebamipide could be considered as one of the adjunctive treatment options in managing renal manifestations in lupus according to the present results from lupus-prone mice.
REFERENCES: NIL.
Acknowledgements: This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: 2022R1I1A1A01068210).
Disclosure of Interests: None declared.