

Background: Unsupervised analysis of gene expression datasets unraveled specific gene signature for systemic sclerosis (SSc).
Objectives: Here, we aimed to identify potential therapeutic targets in SSc utilizing previous gene sets along with machine learning techniques and subsequently validate one lead target functionally in preclinical models.
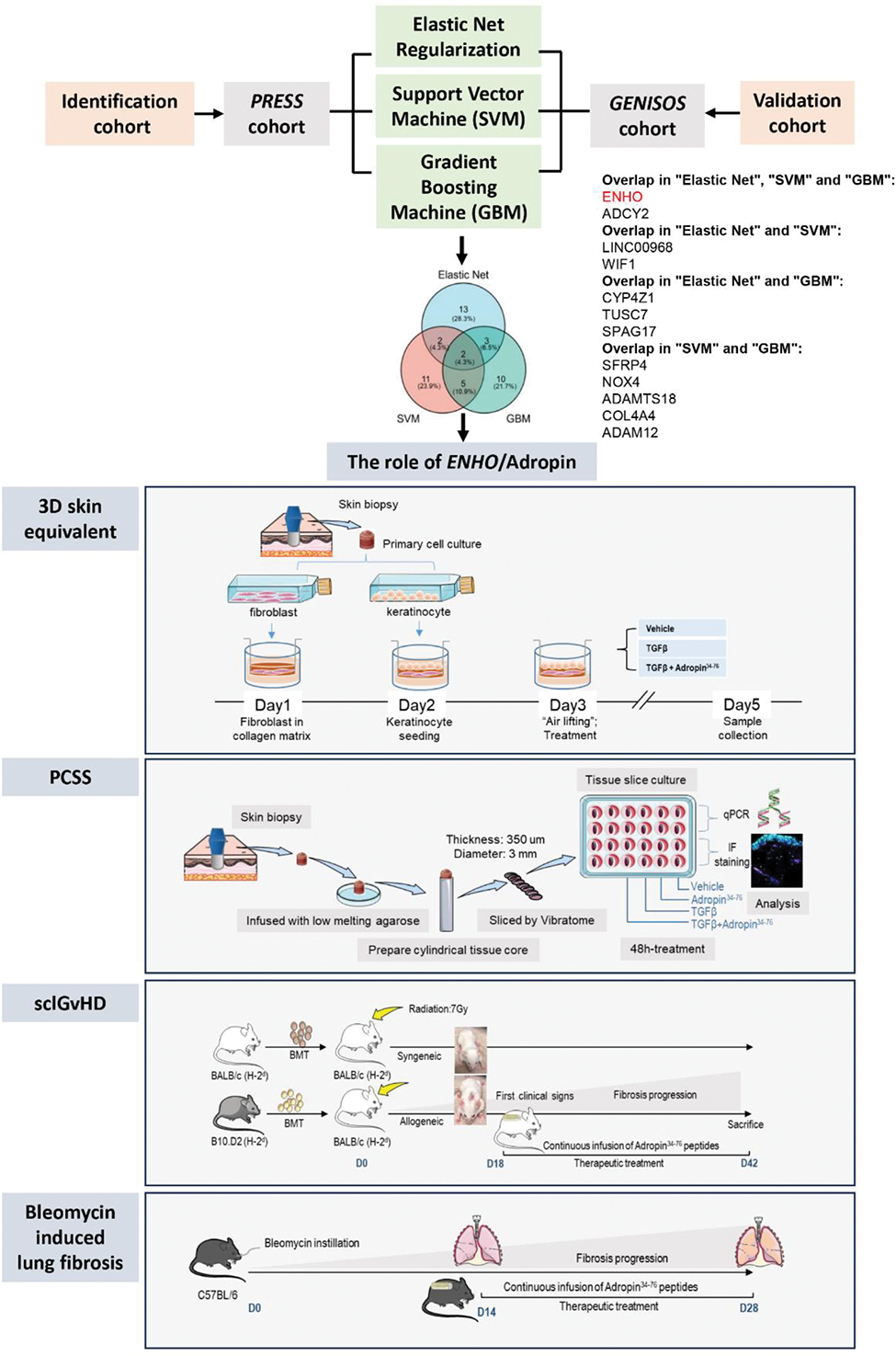
Methods: Three independent machine learning (ML) approaches such as Elastic Net Regularization, Support Vector Machine and Gradient Boosting Machine were used to identify candidate genes regulating aberrant fibroblast activation in SSc. The effects of Adropin (encoded by energy-homeostasis-associated ( ENHO )), identified by ML, were analyzed in multiple in-vivo , in-vitro , and ex-vivo models, including cultured human fibroblasts, 3D full-thickness human skin-equivalents, precision-cut human skin slices and murine models of SSc.
Results: Machine learning based evaluation of bulk RNA-seq datasets identified ENHO as a signature gene for SSc. Two large external cohorts, the PRESS and the GENISOS, as well as our internal cohort, demonstrated downregulation of Adropin/ ENHO in dermal fibroblasts of SSc patients. Functional in vitro experiments demonstrated that TGFβ reduced Adropin/ ENHO expression in a specific JNK2-dependent manner. Bioactive Adropin 34−76 peptides in turn inhibited TGFβ-induced fibroblast activation and subsequent fibrosis, as illustrated in dermal fibroblasts and in 3D full-thickness human skin-equivalents. Treatment with Adropin 34-76 also exhibited an inhibitory effect on the upregulation of αSMA and stress fibers and the deposition of type I collagen in precision-cut human skin slices of SSc. RNA-seq demonstrated profound de-enrichment of categories relevant for the pathogenesis of SSc following the treatment of Adropin 34-76 in SSc derived precision-cut human skin slices, such as extracellular matrix (ECM) remodeling, proinflammatory and profibrotic signaling pathways. Moreover, therapeutic administration of Adropin 34−76 with pre-established fibrosis exerted antifibrotic effects in skin and lungs in mice with sclerodermatous chronic graft-versus-host-disease (sclGvHD) and bleomycin-induced lung fibrosis. Knockdown of GPR19, a putative receptor of Adropin, abrogated the antifibrotic effect of Adropin in fibroblasts. RNA-seq demonstrated that the antifibrotic effects of Adropin 34−76 were functionally linked to Hedgehog signaling and GLI1 deactivation. The inhibitory effects of Adropin 34−76 on GLI1 as a therapeutic mode of action was experimentally confirmed in vitro and in vivo using fibroblast-to-myofibroblast transition assays in combination with ChIP-seq
Conclusion: The expression of Adropin/ ENHO is downregulated in SSc in a TGFβ-dependent manner. Therapeutic application of Adropin 34−76 ameliorates fibroblast activation and tissue fibrosis by inhibition of Hedgehog/GLI1 signaling. Adropin 34−76 might thus offer potential for the treatment of fibrosis.
Graphical Abstract
REFERENCES: [1] Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390:1685-1699. doi: 10.1016/S0140-6736(17)30933-9.
[2] Distler JHW, Gyorfi AH, Ramanujam M, Whitfield ML, Konigshoff M, Lafyatis R. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol. 2019;15:705-730. doi: 10.1038/s41584-019-0322-7.
[3] K. G. Kumar, J. L. Trevaskis, D. D. Lam, G. M. Sutton, R. A. Koza, V. N. Chouljenko, K. G. Kousoulas, P. M. Rogers, R. A. Kesterson, M. Thearle, A. W. Ferrante, Jr., R. L. Mynatt, T. P. Burris, J. Z. Dong, H. A. Halem, M. D. Culler, L. K. Heisler, J. M. Stephens, A. A. Butler, Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell metabolism 8, 468-481 (2008); published online EpubDec (10.1016/j.cmet.2008.10.011.
Acknowledgements: We thank Christoph Liebel, Regina Kleinlein, Wolfgang Espach, Lena Summa, and Vladyslav Fedorchenko for excellent technical assistance. Likewise, we would like to thank Zoltán Winter from the Optical Image Centre (OICE) Erlangen for the support with the acquisition of confocal images and analysis. We also would like to acknowledge the supports from Lisa Nagel at Preclinical Imaging Platform Erlangen (PIPE) for chest CT scan and assessment.
Disclosure of Interests: Jörg Distler JHWD is stock owner of 4D Science and Scientific Leader of FibroCure., Although no disclosures directly relate to the present study, JHWD has consultancy relationships with AbbVie, Active Biotech, Anamar, ARXX, AstraZeneca, Bayer Pharma, Boehringer Ingelheim, Celgene, Galapagos, Genentech, GSK, Inventiva, Janssen, Novartis, Pfizer, Roche and UCB., JHWD has received research funding from Anamar, Argenx, ARXX, BMS, Bayer Pharma, Boehringer Ingelheim, Cantargia, Celgene, CSL Behring, Galapagos, GSK, Inventiva, Kiniksa, Lassen, Sanofi-Aventis, RedX, UCB., Minrui Liang: None declared.