

Background: Patients with systemic sclerosis (SSc) are known to have a high cardiovascular (CV) and cerebrovascular (CVB) risk [1]. In the context of SSc, microangiopathic manifestations like digital ulcers, nailfold capillaroscopic abnormalities, and pulmonary arterial hypertension (PAH) have been thoroughly examined. However, data on macrovascular involvement and accurate assessment tools of individual CV/CVB risk are scarce [3,4].
Objectives: This study aimed to examine gold standard surrogates of CV risk and subclinical atherosclerosis, along with novel Doppler-sonographic markers of CVB risk and a retinal vessel analysis (RVA) parameter in a large cohort of patients with SSc (Systemic SCLEROsis CARDiovascular risk cohort; SCLEROCARD). Moreover, it sought to evaluate correlations of these markers with disease- and patient-associated characteristics, as well as with traditional CV risk factors.
Methods: The SCLEROCARD study focuses on CV/CVB-risk examination in patients treated for SSc (EULAR/ACR-criteria) in a multicenter study setting. Patients and healthy controls underwent oscillometric and B-mode ultrasound (US) examinations (aortic stiffness, carotid-Intima-Media-Thickness, qualitative & quantitative plaque assessment), as well as novel Doppler-US-assessments [resistance- (RI) & pulsatility- (PI) indices and flow velocity integral (FVI)] of the common carotid arteries (CCA). Moreover, retinal vessel analysis (RVA) was performed via a static RVA model to determine the ophthalmic arteriovenous-ratio (AVR) and the central retinal arterial/venous equivalents (CRAE, CRVE).
Results: A total of 202 SSc patients [85.1% ♀, 58 years (51–67, IQR)] and 80 control subjects [86.3% ♀ 50.5 years (35.23–57.75, IQR)] were recruited (Table 1). In the patient group, cfPWV values were statistically significantly higher compared to controls [8.09 m/s (6.71–9.14, IQR) vs. 6.85 m/s (5.93–7.7, IQR); p<0.001], even after adjustment for the effect of confounding factors (p adj = 0.002). Similarly, cIMT [0.92 mm (0.8–1.1, IQR) vs. 0.83 mm (0.65–0.89); p<0.001] and subclinical atherosclerosis incidence (79.4% vs. 33.3%; p<0.001) were found to be significantly higher in the patient group, respectively. Interestingly, cfPWV was significantly higher in patients with PAH (rho=0.267; p=0.007) and correlated moderately/poorly with erythrocyte sedimentation rate (rho=0.216; p=0.005). Moreover, cfPWV and cIMT associated significantly among SSc-Patients with several traditional CV risk factors, such as mean arterial pressure (rho=0.404; p<0.001), hyperlipidemia (rho=0.201; p=0.006), and type II diabetes (rho=0.329; p<0.001) (all; cfPWV), as well as the presence of arterial hypertension (rho=0.278/ 0.331; both p=0.001) (cfPWV and cIMT).
Central retinal vein diameter correlated with CRP (rho=0.536; p=0.039). Interestingly, pathologic RVA values and the retinal arterial-venous ratio correlated with muscle involvement (myositis) in the context of SSc (rho=0.461; p=0.036 and rho=0.489; p=0.021, respectively).
Conclusion: This study represents one of the most extensive investigations into CV surrogate markers within the SSc field. It is moreover the first study highlighting the potential diagnostic role of cfPWV in screening for PAH among SSc patients. Our findings demonstrate that individuals with SSc exhibit significantly increased aortic stiffness and more pronounced carotid atherosclerosis compared to healthy controls, suggesting a heightened CV/CVB risk.
To summarize, cfPWV and carotid sonography may prove valuable in early identification of SSc patients at high risk for CV events and PAH.
REFERENCES: [1] Cen X, et al. Medicine. 2020;99(47):e23009.
[2] Triantafyllias, K., et al. Diagnostics. 2023;13(11),1870.
[3] Triantafyllias K, et al. Clin Exp Rheumatol. 2019;37(6):994-1002.
Table 1. Descriptive characteristics.
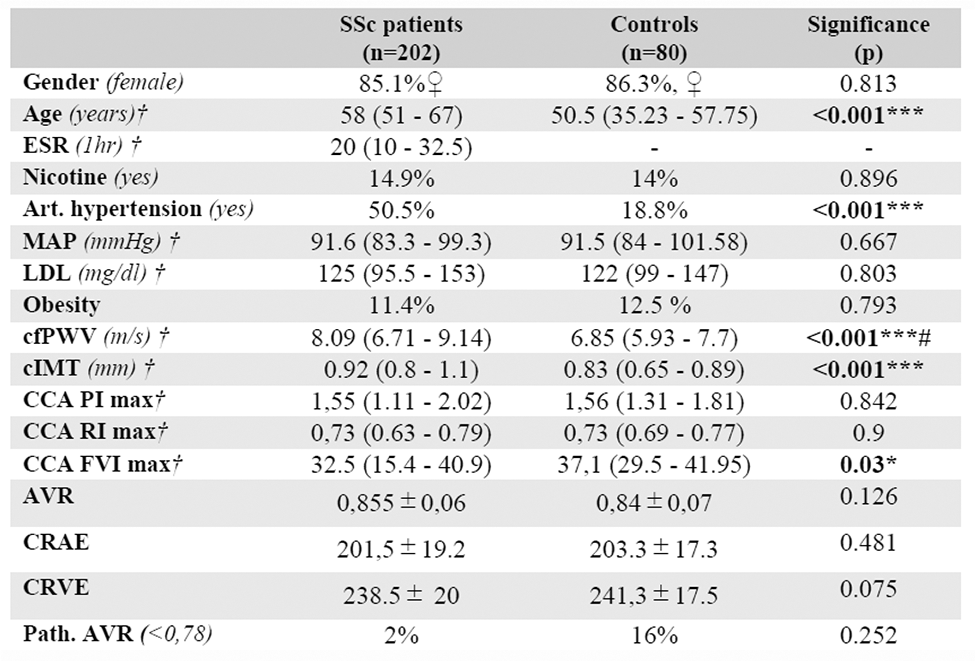
*p-value < 0,001; ***p-value <0.001; #adjusted p-value#
ESR= erythrocyte sedimentation rate; MAP= mean arterial pressure; ACC-PI/RI= common carotid artery pulsatility-index/ resistance index; AVR= arteriovenous ratio; CRAE= central retinal artery equivalent; CRVE= central retinal vein equivalent
Acknowledgements: NIL.
Disclosure of Interests: Konstantinos Triantafyllias Pfizer, Novartis, Galapagos, UCB, Janssen, Chugai, Sanofi, AbbVie, Pfizer, Galapagos, Anna Raetsch: None declared, Andreas Schwarting: None declared.