

Background: Significant progress has been achieved over the course of recent time in elucidating the complex underlying pathophysiological mechanisms of ANCA associated vasculitis (AAV). Despite these breakthroughs in research, the range of laboratory parameters available for evaluation in everyday clinical practice to guide the diagnostic process and management of patients with AAV remains very limited. Much effort is nowadays being expended on identifying such prospective biomarkers and assessing their potential for clinical application.
Objectives: To evaluate immune cell (sub)populations of patients with AAV and identify significant differences between patient group and healthy controls, and subsequently to also identify differences between cytometric profiles between subgroups of patients based on overall disease severity, specific organ involvement and presence of disease relapse.
Methods: A panel of immune cell (sub)populations was investigated in peripheral blood samples from patients with newly confirmed diagnosis of AAV (n=52) and healthy controls (n=16). Patients with AAV were evaluated based on their initial clinical presentation using the validated Birmingham Vasculitis Activity Index version 3 (BVAS v3). For further analysis, patients were divided into subgroups based on overall disease severity (two groups defined as less severe and more severe by group median BVAS v3 score of 15), presence of particular organ domain involvement, and presence of identified disease relapse later on in the course of the condition requiring treatment reevaluation. Smaller subgroups significant by their clinical severity, namely patients requiring long-term hemodialysis due to AAV-conditioned organ damage (n=6) and patients with lethal course of disease (n=5), were also evaluated. Statistical analysis tools (Mann-Whitney, Spearman correlation, regression analysis) were used to evaluate data obtained.
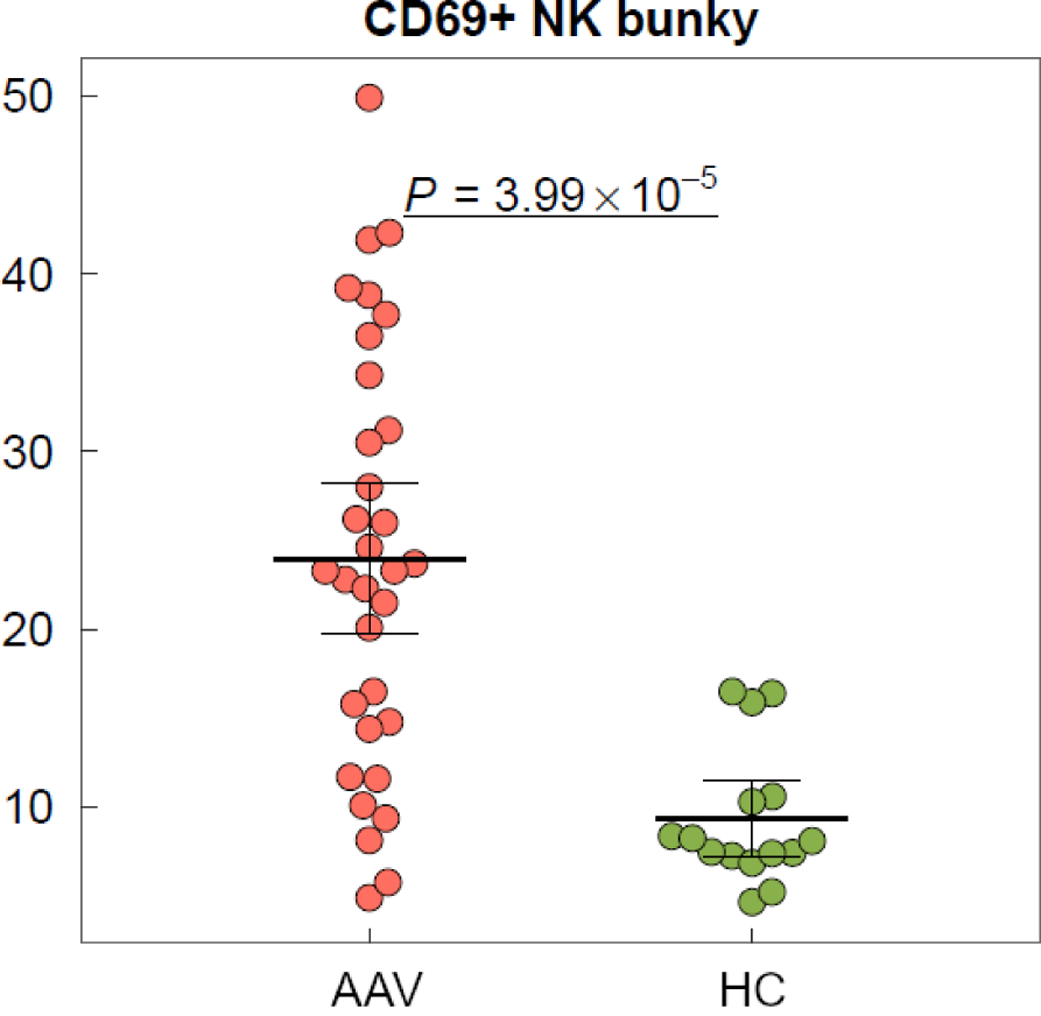
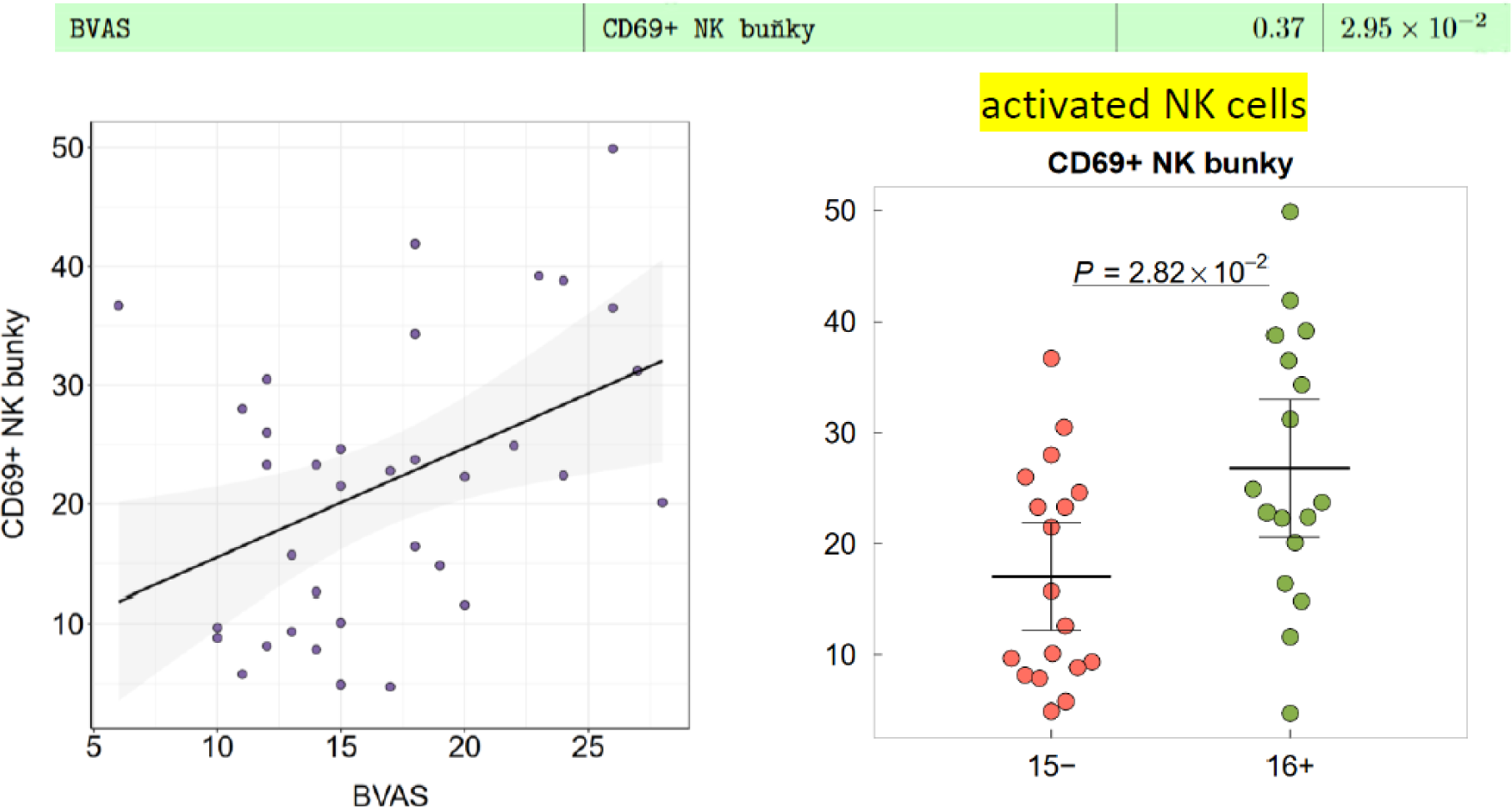
Results: Preliminary analysis reveals significant differences between immune cell populations between studied groups and subgroups – among them non-specific upregulation of peripheral neutrophil granulocytes (p=9.28 ×10 -8 ) and suppression of peripheral lymphocytes (p=2.6×10 -8 ) and monocytes (p=2.06×10 -6 ) in AAV patients compared to healthy controls. Similarly, particular immune cell subpopulations of CD 69+ NK cells (p=3.99 ×10-5) and CD 69+ CD 8+ T-lymphocytes (p=0.008) were highly expressed in AAV patients, whereas subpopulation of nonclassical monocytes was significantly reduced in AAV patients in comparison to healthy controls (p=2.81 ×10 -7 ). These trends were also consistently observed in the comparison between subgroups defined by disease severity as described above, with patients presenting with more severe form of disease having further significantly elevated both CD 69+ NK cells and CD 69+ CD8 + T-lymphocytes (p=0.025, p=0.029) compared to patients with less severe AAV.
Conclusion: Significant flow cytometry immune cell profile differences can be observed between groups of patients with diagnosed AAV in comparison to healthy controls, as well as between subgroups of patients based on disease severity and organ involvement. Further inquiry into these differences is warranted, as they may offer potential for clinical application in precising diagnosis, assessing disease severity and possibly guiding treatment choices or even present possible therapeutic targets.
REFERENCES: NIL.
Acknowledgements: Supported by MH CZ – DRO (FNOL, 00098892) and by IGA_LF_2023_002.
Disclosure of Interests: Jakub Videman: None declared, Adela Skoumalova: None declared, Marketa Dudkova: None declared, Martina Skacelova Eli Lilly, Novartis, AbbVie, Sobi, UCB, Anna Petrackova: None declared, Zuzana Mikulkova: None declared, Eva Kriegova: None declared, Pavel Horak Eli Lilly, Novartis, AbbVie, Sobi, AstraZeneca, Boehringer, UCB, Sandoz, Zentiva.