

Background: Takayasu arteritis (TAK) is a rare large vessel vasculitis where no immunosuppressive drug has yet been proven effective in a randomized controlled trial. Therefore, it is necessary to explore newer therapies in TAK. Arterial wall inflammation leads to fibrosis in TAK, thereby leading to arterial stenosis [1]. The polyphenolic isoflavone Genistein may be useful as an adjuvant therapy in TAK due to its anti-oxidant, anti-inflammatory, and cardioprotective properties [2].
Objectives: To explore the pharmacological mechanism of action of genistein in the treatment of TAK using network pharmacology and molecular docking.
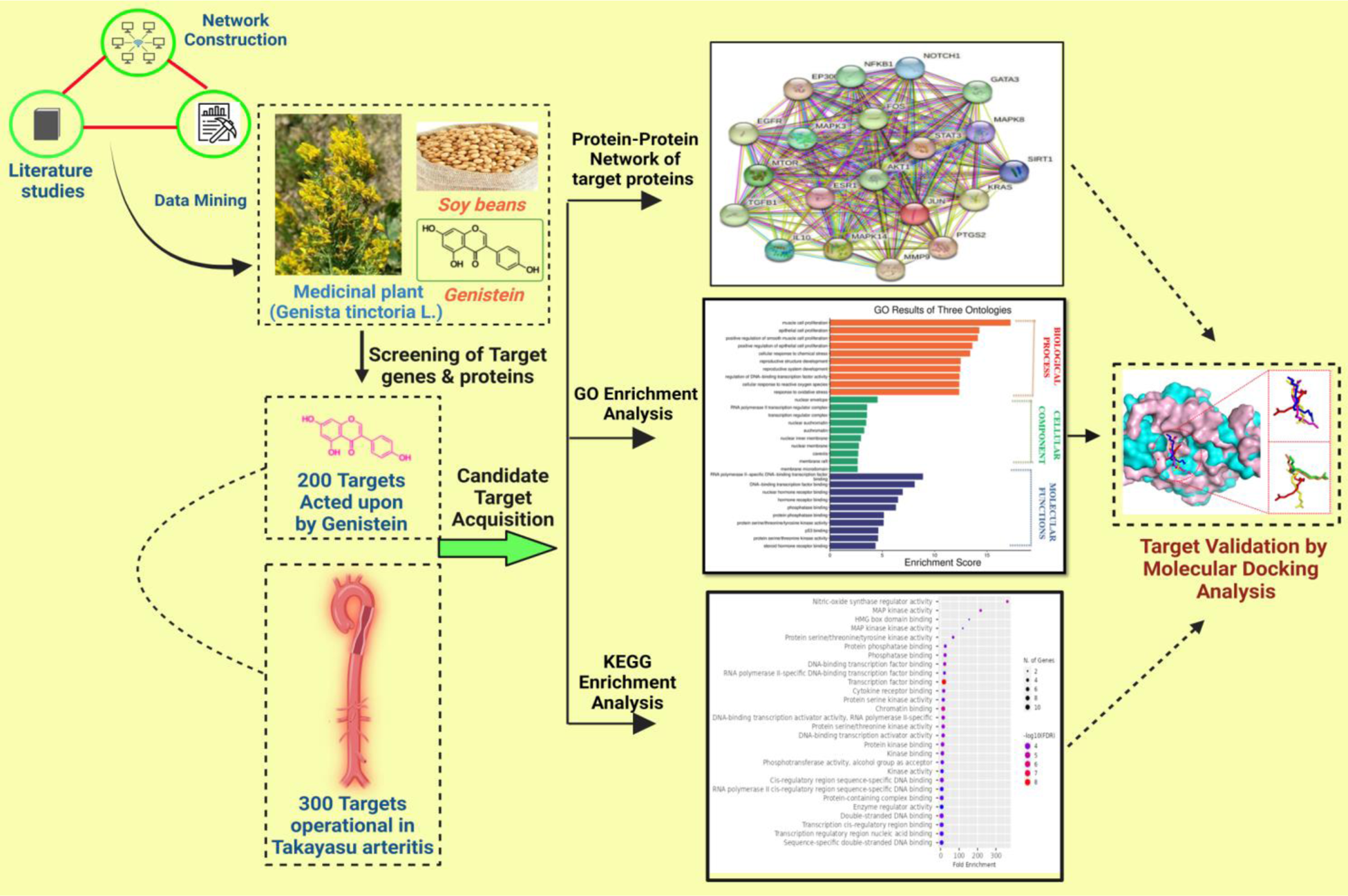
Methods: We searched open-access databases for genes and proteins linked to TAK and anticipated to be Genistein targets by using NCBI, Therapeutic Target and Binding Databases. We used STRING database with a medium confidence criterion set at >0.4 to identify protein-protein interactions and build a regulatory network for crossover genes. Cytoscape software was used to filter out top 20 hub targets, along with their cluster analysis by CytoHubba plugin tool. A p value<0.05 and q value<0.05 significance threshold was used to filter out enriched categories, along with degree of enrichment using log10 transformed p value. Enrichment analysis was done for biological, molecular processes and cellular component using GO Enrichment Analysis tool. Thereafter, construction of drug-compound-target-pathway-disease network was done to validate the pharmacological network of hub targets by Shiny GO 0.77 tool. Binding mode of Genistein with target proteins were evaluated by Molecular Docking via AutoDock Vina and hydrogen bond interactions were assessed by Biovia Discovery studio visualizer and Pymol tool.
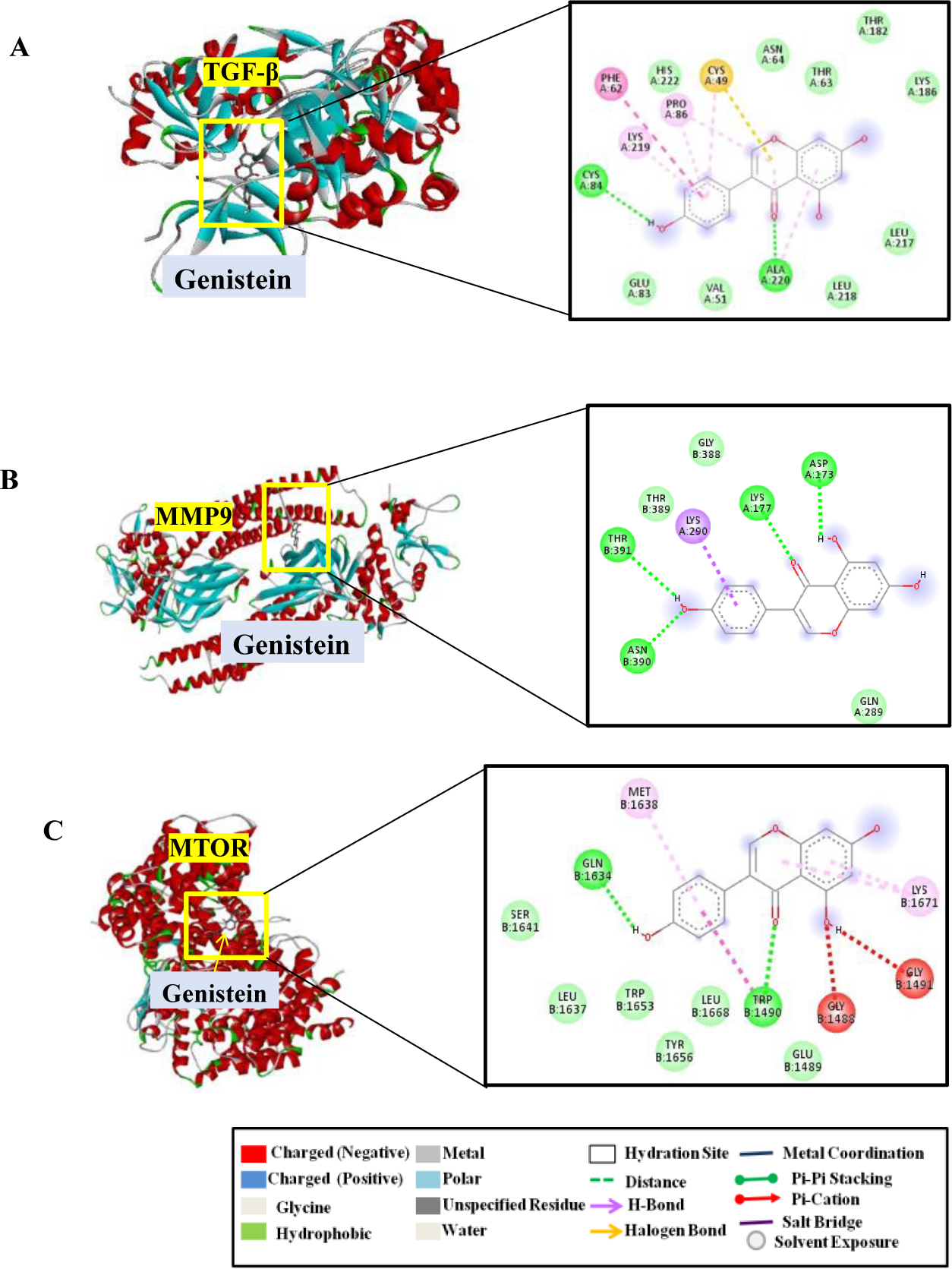
Results: We identified 500 potential therapeutic targets, amongst which 20 were hub targets for Genistein. The protein targets-MAPK3, STAT3, JUN, AKT1, NF-kB, MAPK3, TGF-β, ESR1, EGFR, MAPK14, MTOR, MMP9, IL10 were present in both Genistein target networks and TAK-associated pathways that regulate Th1 and Th2 differentiation, T-cell receptor signaling, FOXO, Th17-IL-17 signaling pathway, immune cell trafficking, cell-to-cell signaling and inflammatory response (Figure 1). Considering the lowest binding energy (ΔGb, Kcal/mol) and hydrogen bond interaction, amongst the top 10 protein targets, TGF-β, MMP-9 & MTOR (drivers of both vascular inflammation and vascular fibrosis in TAK) showed strong affinity with Genistein having binding energies -8.4 kcal/mol,−8.3 kcal/mol, and -9.4 kcal/mol respectively. The inhibitor Genistein showed strong hydrogen bond interactions with amino acid residues Phe-62, Pro-86 and Lys-219(in TGF-β), Lys-177 and Asp-173(in MMP-9) and Met-1638, Trp-1490 and Lys-1671(in MTOR).These interactions contribute towards the stability of Genistein with their target proteins (Figure 2).
Conclusion: Genistein inhibits the pro-fibrotic proteins (TGF-β, MMP9 & MTOR) and affects various inflammatory pathways known to be operational in Takayasu arteritis, including Th1 and Th17 related pathways. There is a rationale to evaluate Genistein in pre-clinical studies and thereafter in clinical trials of Takayasu arteritis.
REFERENCES: [1] Misra DP, Singh K, Sharma A, Agarwal V. Arterial wall fibrosis in Takayasu arteritis and its potential for therapeutic modulation.. Front Immunol 2023;14:1174249. 10.3389/fimmu.2023.1174249.
[2] Jafari S, Shoghi M, Khazdair MR. Pharmacological Effects of Genistein on Cardiovascular Diseases. Evid Based Complement Alternat Med. 2023;2023:8250219.
Genistein inhibits pro-fibrotic proteins (TGF-β, MMP9 & MTOR) and affects various inflammatory pathways known to be operational in Takayasu arteritis, including Th1 and Th17 related pathways.
Molecular docking of Genistein with TGF-β, MMP9 & MTOR: Binding of Genistein inhibitor with residues of TGF-β(A), MMP-9(B) and MTOR(C) with 2D diagram showcasing intermolecular interactions of Genistein with TGF-β, MMP-9 and MTOR. (Ligand is denoted in grey cartoon form and active site of receptor in multicolour domain)
Acknowledgements: The work is funded by Department of Science & Technology (DST)-Innovation in Science Pursuit for Inspired Research (INSPIRE) Fellowship (INSPIRE Code IF210497).
Disclosure of Interests: None declared.