

Background: Prime-boost vaccination combining 13-valent pneumococcal conjugate vaccine (PCV13) followed by 23-valent polysaccharide vaccine (PPSV23) after 8 weeks is recommended for immunosuppressed patients with rheumatic diseases. There is limited data related to immunogenicity and safety of this strategy in patients with psoriatic arthritis (PsA) and systemic sclerosis (SSc).
Objectives: To evaluate the immunogenicity and safety of pneumococcal prime-boost vaccination in patients with PsA and SSc at week 14 with a one-year follow-up.
Methods: We prospectively assessed the immunogenicity of prime-boost vaccination in patients with PsA and SSc by measuring all serotype-specific pneumococcal immunoglobulin G (IgG) antibody concentrations at baseline (prior to PCV13), 8 weeks post PCV13, 6 weeks after PPSV23 (provided 8 weeks after PCV13) (primary outcome), and 1-year post-PCV13 vaccination as well as vaccination safety and rheumatic disease activity. Good response to vaccination was defined as a post-vaccination IgG concentration ≥1.3 μg/mL for 70% of the measured serotypes, the 13 serotypes included in the PCV13, and 10 exclusive PPSV23 serotypes. Univariate analysis was performed to identify the predictors for vaccination response.
Results: A total of 35 patients with PsA (females n=17, 49%, mean age 53±14 years, mean disease duration 15.5±13 years) and 22 SSc patients (females n=20, 91%, mean age 54±17 years, mean disease duration 12.3±11.5 years) with diffuse (n=17) and limited (n=5) forms were included, of whom 11 PsA and 9 SSc patients were vaccinated with PPSV23 before the enrollment into the study. Thirteen (68.6%) PsA patients were treated with methotrexate (MTX) and 24 (68.6%) with anti-cytokine biologics; a total of 6 (27%) SSc patients were treated with mycophenolate, 3 (13.6%) with MTX, one (4.5%) with IL-6, and one (4.5%) with rituximab. Ten SSc and 18 PsA patients completed 1-year follow-up.
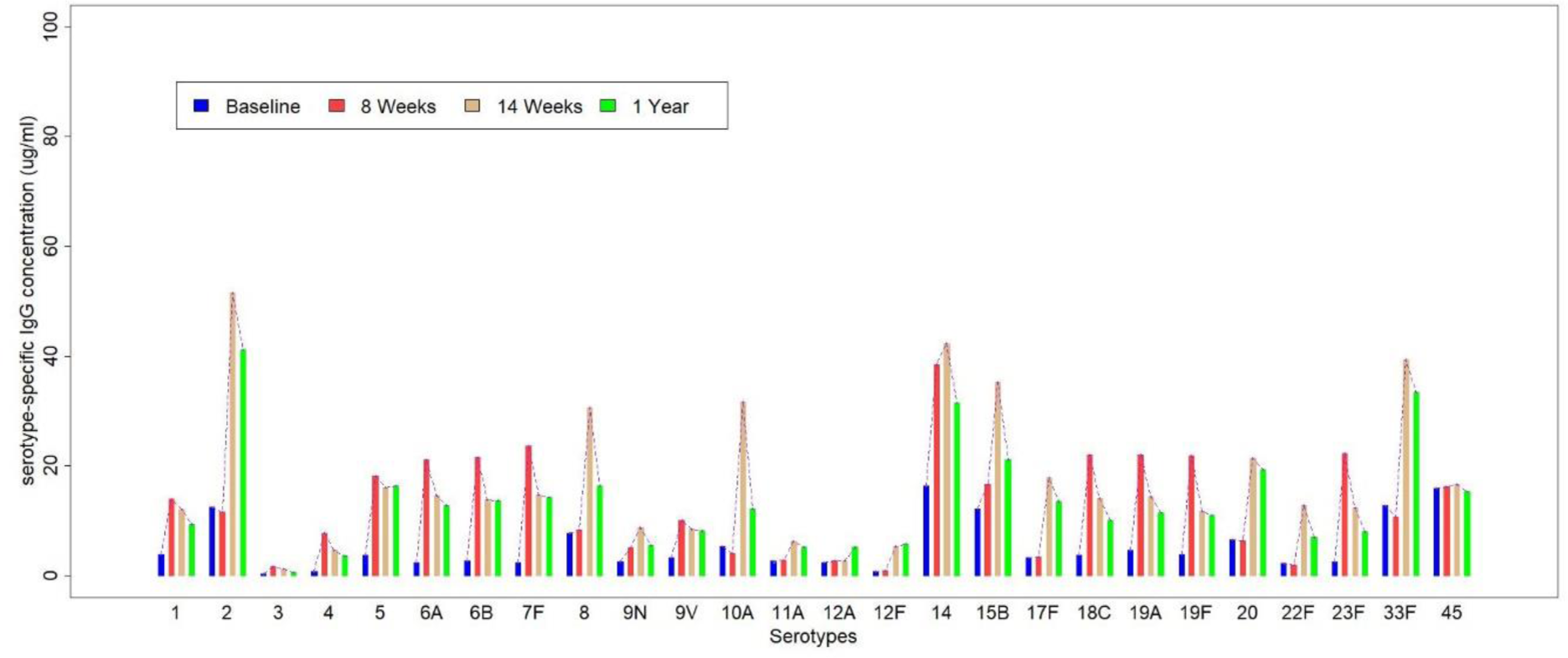
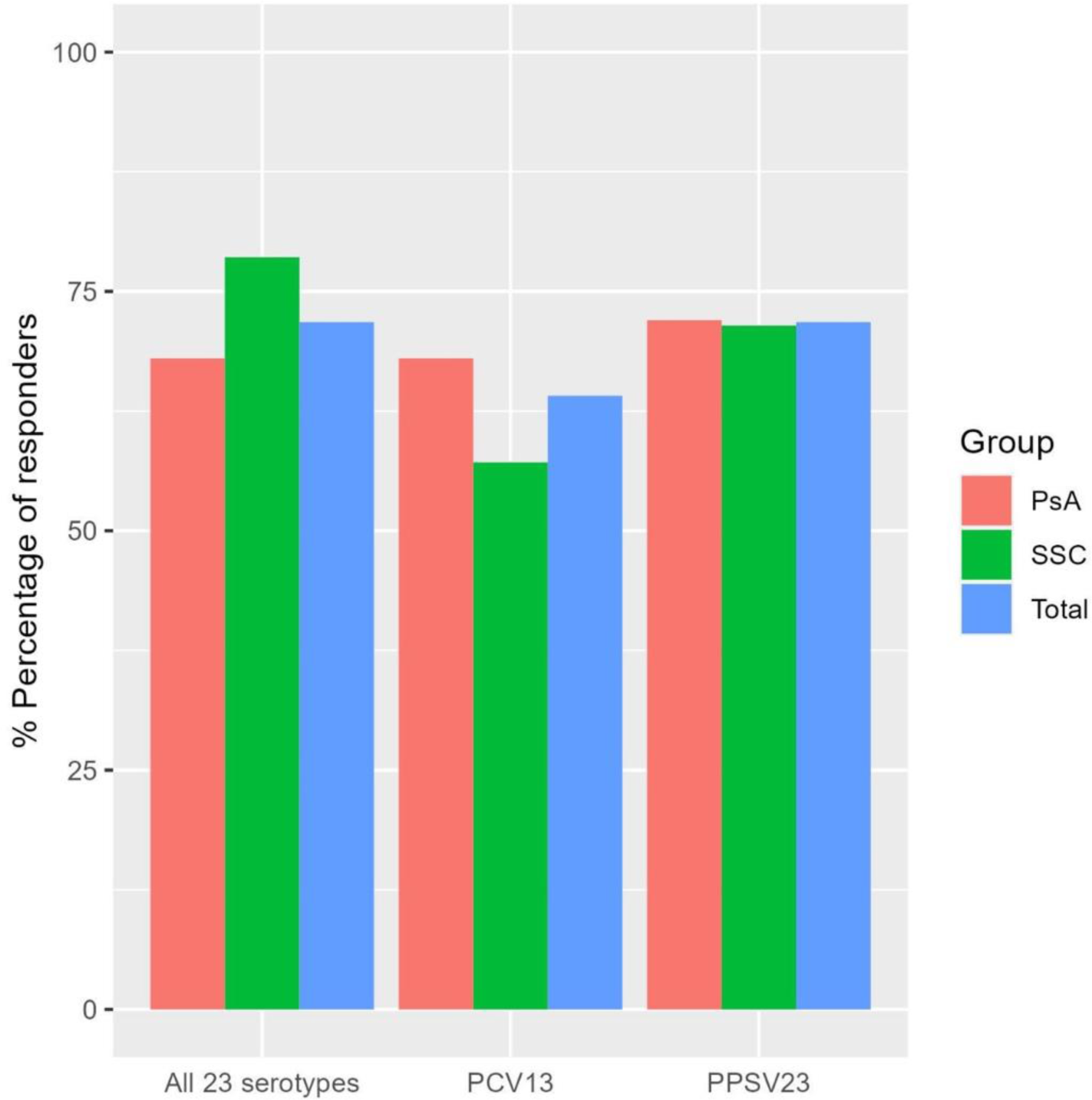
Following prime-boost vaccination, there was a respective increase of all the vaccine-specific serotypes (Figure 1). Overall, most of the cohort (n=28, 72%) developed a good immunogenic response to prime-boost vaccination for all the 23 serotypes at week 14 with a comparable response rate in PsA and SSc patients (n=17, 68% and n=11, 78.6%, respectively) (Figure 2).
Vaccination with PPSV23 following PCV13 did not have a boosting effect on the PCV13 serotypes, with serotype 14 being the only exception. Over 1 year since PCV13 vaccination, IgG concentrations slowly decreased but were still higher compared to baseline. The immunogenic response for all serotypes was comparable in patients with SSc and PsA, except for a significantly higher response of the serotypes 18C and 33F in patients with PsA. No serious side effects were observed during the study and post-vaccination disease activity remained stable in the majority of patients through all the study time-points. Univariate analysis did not identify any predictors for the positive immune response to vaccination.
Conclusion: Overall, both patients with SSc and PsA developed an adequate immunogenic response to the prime-boost vaccination with a good safety profile. Vaccination with PPSV23 following PCV13 did not have a significant boosting effect on the PCV13 serotypes in this study cohort.
REFERENCES: NIL.
Serotype-specific IgG antibody concentrations at baseline (pre-PCV13), 8 weeks post-PCV13, 14-weeks post PCV13 (=6 weeks post-PPSV23), 1-year post-PCV13
Seroconversion rates of all 23 pneumococcal serotypes, the 13 serotypes covered by PCV13, and the 10 serotypes covered only by PPSV23 following the prime-boost vaccination at week 14.
Acknowledgements: NIL.
Disclosure of Interests: None declared.