

Background: Vaccination against pneumococcus reduces the risk of infective events, hospitalisation, and death in individual with inflammatory arthritis, particular in those on immunomodulating therapy who are at risk of worse outcomes from pneumococcal disease
Objectives: To Investigate the serological protection against pneumococcal serovars over time.
Methods: This was a single centre, retrospective cohort study of individuals with rheumatoid arthritis, psoriatic arthritis or axial spondylarthritis who had previously received the PPSV23 polysaccharide pneumococcal vaccine (Pneumovax). Individuals were followed up from January 2021 to August 2023. Dates of previous pneumococcal vaccination were identified using linked primary care records. Serum serotype levels were collected. The primary outcome was serological response defined as a titre ≥0.35mcg/mL in at least five a total of 12 evaluated pneumococcal serovars, examined using the Luminex laboratory platform. A multivariate logistic regression model adjusting for age, gender, ethnicity, co-morbidities and the use of prednisolone, conventional synthetic and biological DMARDs was used to determine the odds of a sustained serological response according to time categorised into ≤5 years, 5-10 years, and ≥10 years since vaccination.
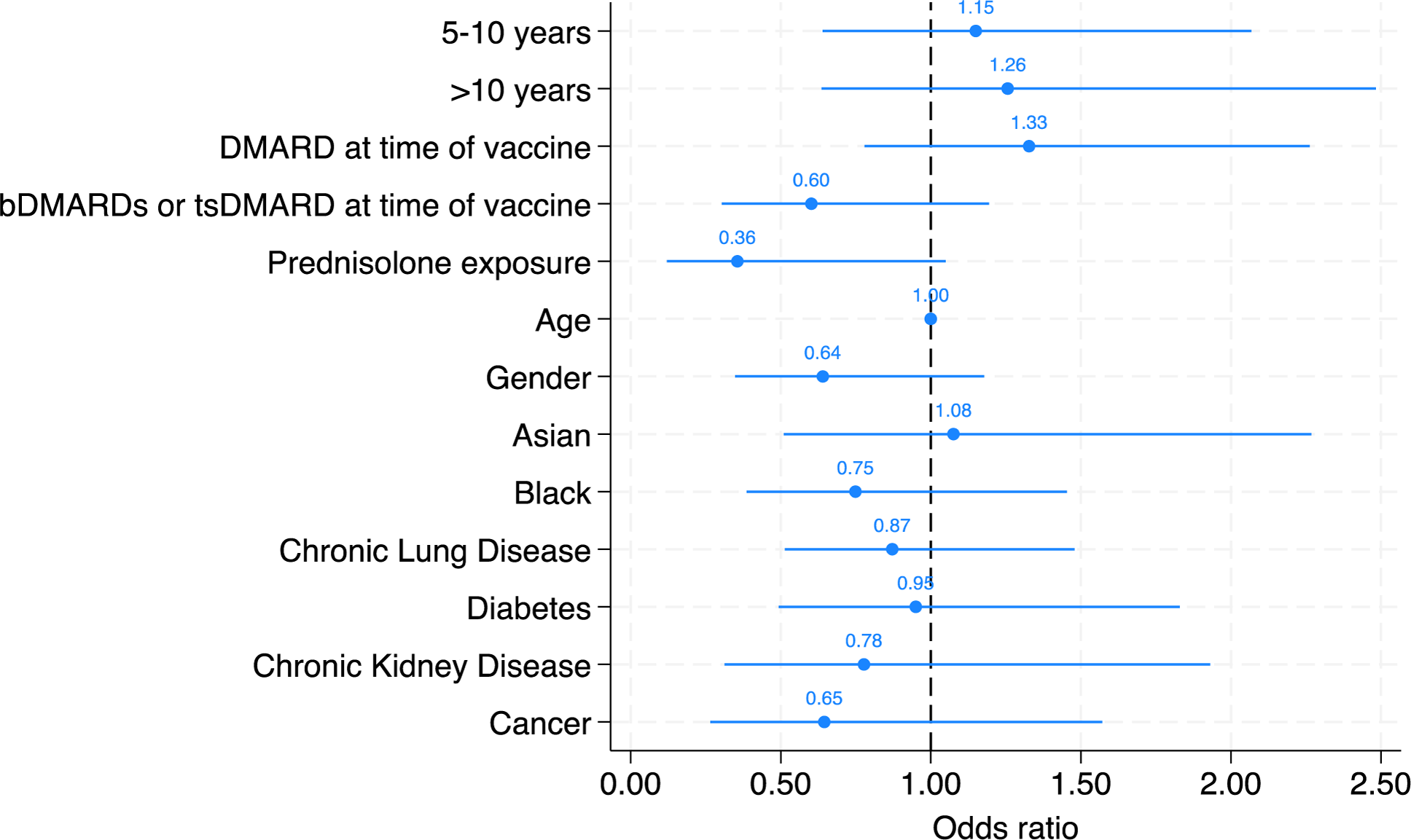
Results: Serological response was measured in 296 individuals with inflammatory arthritis, with rheumatoid arthritis the most common diagnosis (75% of patients). A vaccine response was observed in 195 (66%) patients. The median time between pneumococcal vaccine administration and serological assessment was 6 years (interquartile range 2.4 to 9.9). A positive serological response to at least 5 serovars was present in 195/296 (66%) of patients. Time since vaccination did not significantly associate with serological response [compared to those vaccinated <5 years, the adjusted ORs of vaccine response was 1.15 (95% CI 0.64 to 2.07) in those 5-10 years and 1.26 (95% CI: 0.64 to 2.48) in those vaccinated over 10 years ago. No individual variable from the multivariate model reached statistical significance as an independent predictor of vaccine response, although sensitivity analyses of individuals serovars and restricted to individuals who had not received a booster dose (n=287, 97%) identified a negative association with prednisolone (adjOR 0.30 (95% CI: 0.10 to 0.93).
Conclusion: We have demonstrated that antibody titres following do not appear to wane over time, and it appears more critical to focus on maximising the initial vaccine response, which is known to be diminished in this patient population.
REFERENCES: NIL.
Multivariate logistic regression model demonstrating the likelihood of vaccine response based on time since vaccine, DMARD, bDMARD and tsDMARD use at time of vaccine, prednisolone exposure, age, gender, ethnicity, and co-morbidities. The odds ratio (OR) for each parameter is illustrated in the figure.
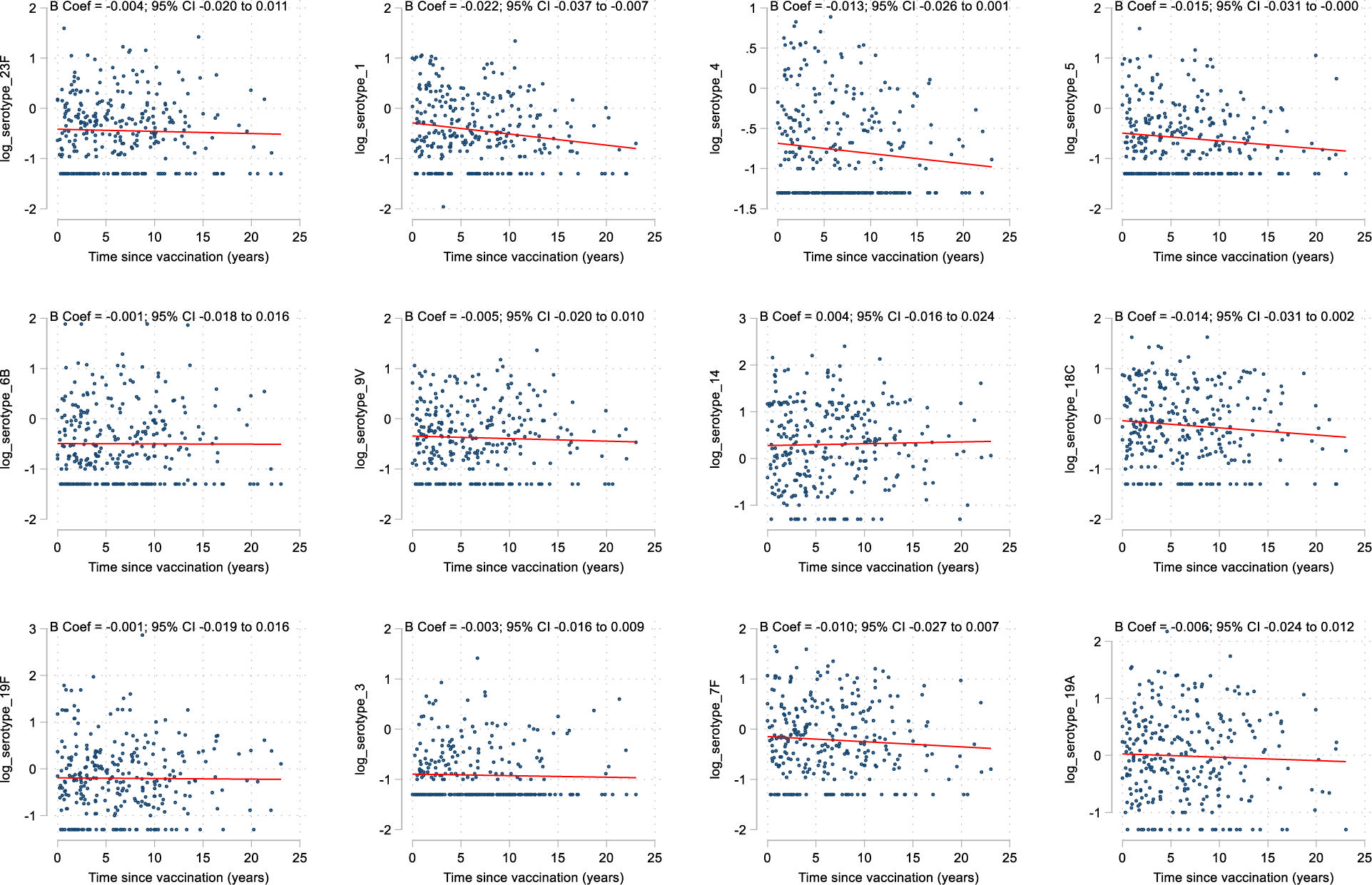
Scatter plot for each individual serotype demonstrating the log transform serotype response against time in years, with a line of best fit derived from a linear regression plot.
Acknowledgements: Centre for Rheumatic Disease, KCL.
Disclosure of Interests: Katie Bechman pfizer, Deepak Nagra abbvie, Christopher baldwin: None declared, Maryam A. Adas: None declared, Mark Russell lilly, Ioasaf Karafotias: None declared, Mark Gibson: None declared, Samir Patel: None declared, rutvi shah: None declared, Zijing Yang: None declared, Edward Alveyn: None declared, Sam Norton: None declared, James Galloway JG has received honoraria from AbbVie, Biovitrum, BMS, Celgene, Chugai, Galapagos, Gilead, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, Sobi and UCB.