

Background: The use of biosimilars in Europe has increased since their introduction in 2006. Biosimilars help to manage drug spending budgets and improve patient access by providing effective and safe treatment options in several disease spaces at a cost-effective price [1] .
Objectives: To evaluate the cost-savings associated with increased use of adalimumab biosimilars and the launch of a tocilizumab biosimilar in 7 European countries: United Kingdom (UK), France, Germany, Spain, Italy, Austria, Ireland, and Sweden. This study also explored expanded access due to the direct cost-savings.
Methods: Ex-ante analyses methods were used to estimate the budget savings and expanded access for each country. Both the adalimumab model and tocilizumab model were parameterized using country-specific data (list price, unit volumes annually, and market shares). Unit sales and dosing regimens were used to estimate the number of adalimumab and tocilizumab users. Two market share conversion scenarios were tested for each country and model. The first market share scenario for the adalimumab model assessed the mid-point between current-day market share (mid conversion) and 100%, the tocilizumab model used the current-day adalimumab market share for each country (mid conversion). The second market share scenario evaluated the savings of exclusive biosimilar use (ie, 100% market share). The tocilizumab model used three discounting scenarios (20%, 30%, and 40%) to determine the cost of a tocilizumab biosimilar. Weighted adalimumab biosimilar prices (based on market share) were used in the adalimumab model.
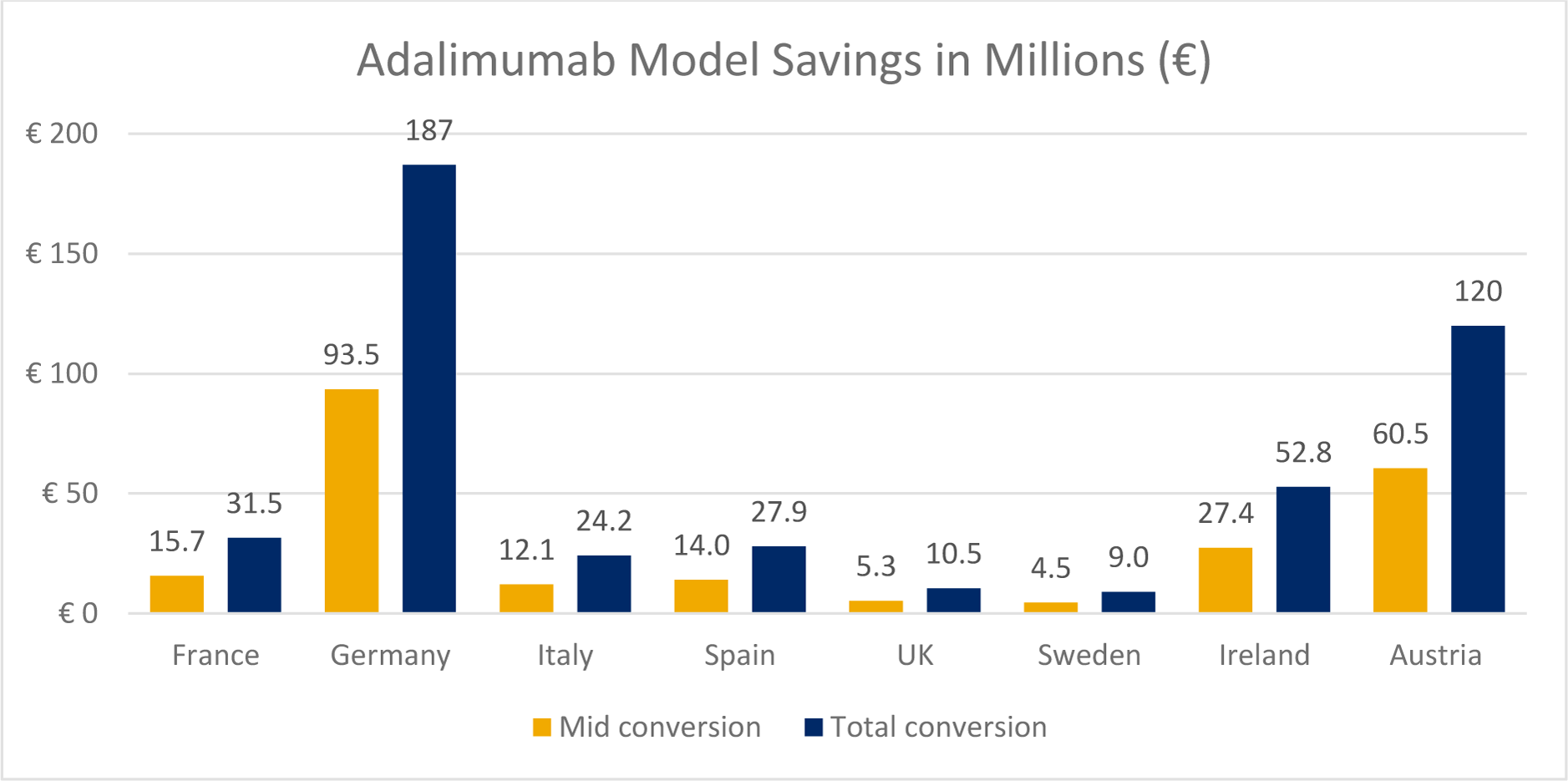
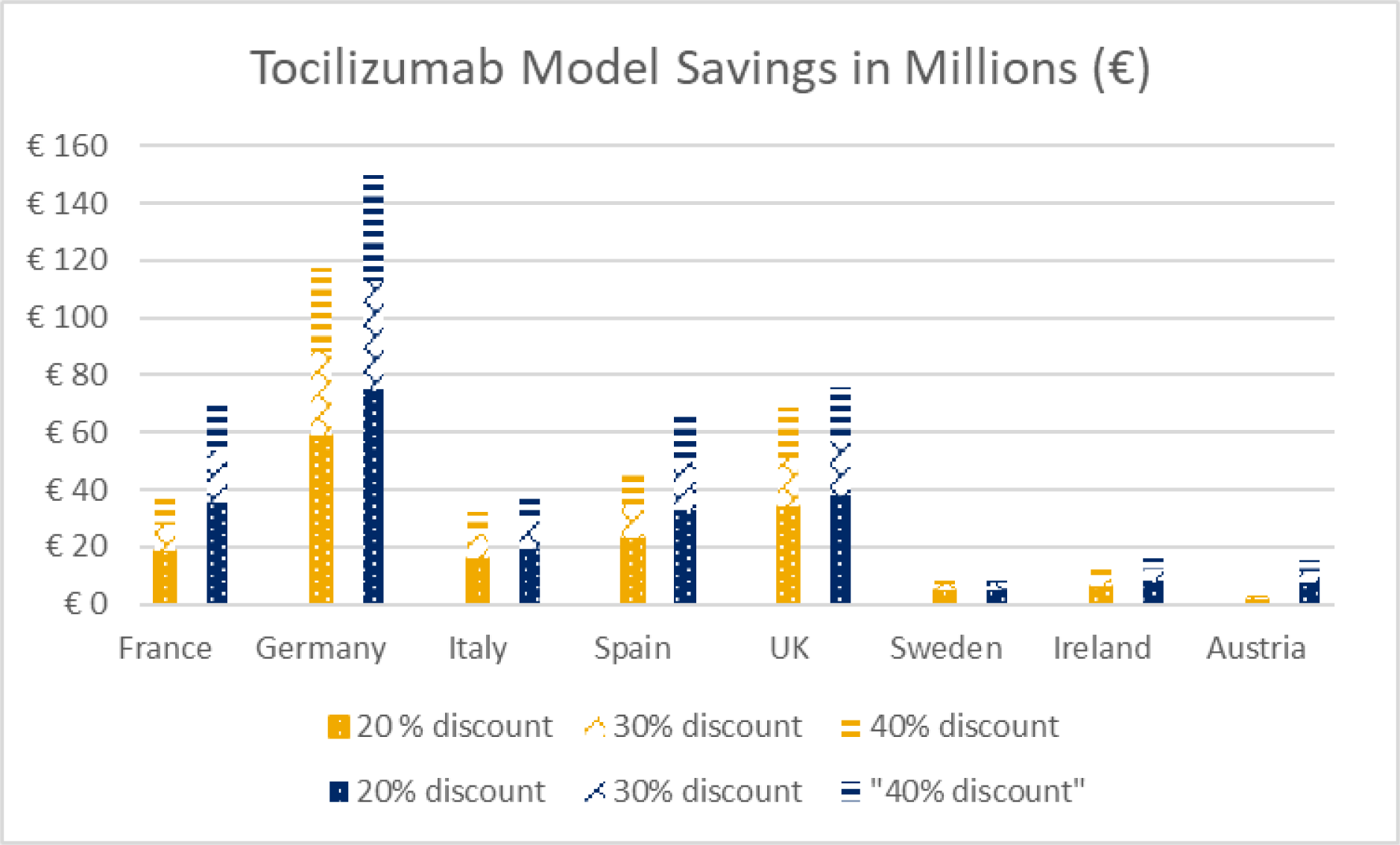
Results: The cumulative savings and expanded access due to exclusive adalimumab biosimilar use across all 7 countries was over €462 million and 65,593 additional patients, respectively. In Austria, the country with the lowest current-day market share (19%), an increase to 60% market share resulted in a savings of €129 million with 12,930 additional patients treated. In the UK, the country with the highest current-day use of adalimumab biosimilars (90% market share), exclusive use of biosimilars still yielded meaningful savings of €10.5 million (1,096 additional patients treated). Germany had the highest current-day drug cost (greater than €1 billion despite 78% biosimilar use). An increase to 89% market share could provide savings of €93.5 million (9,727 additional patients treated). Overall, the savings associated with exclusive use of adalimumab biosimilars ranged from €9.0 million in Sweden to €186 million in Germany. In the tocilizumab model, the most conservative scenario (20% discount) yielded a savings of €1.5 million in Austria to €58.7 million in Germany corresponding to 143 (representing a 4.8% increase) to 3,705 (a 19.5% increase) in additional patients treated. The cumulative savings and additional patients treated under the most conservative scenario were €163 million and 13,818 respectively. In Germany, increasing the current day market share to 100%, yielded savings up to €150 million with 12,667 additional patients treated. In France and the UK, a 100% conversion to tocilizumab biosimilar (at 40% discount) could create up to €71.5 and €75.9 million in savings yielding 14,167 and 10,000 additional patients treated, respectively. In Sweden and Ireland, countries with a lower tocilizumab population, savings of €10.4 and €15.8 million were observed in the 100% market share and 40% discount scenario, respectively. In both Sweden and Ireland, savings could allow 1,333 additional patients treated. Overall, exclusive use of a tocilizumab biosimilar at a 40% discount created €444 million in savings (51,333 additional patients treated).
Conclusion: The increased use of adalimumab biosimilars can provide additional savings and expanded treatment access. Moreover, the introduction of a tocilizumab biosimilar can be beneficial to several markets in Europe.
REFERENCES: [1] IQVIA (2021). The impact of Biosimilar Competition in Europe [White Paper].
Acknowledgements: NIL.
Disclosure of Interests: Kunal Shastri is an employee of Fresenius Kabi, Kerise Clarke is an employee of EVERSANA, a company which has received consulting fees from Fresenius Kabi, Margaret Ainslie-Garcia is an employee of EVERSANA, a company which has received consulting fees from Fresenius Kabi, Nicole Ferko is an employee of EVERSANA, a company which has received consulting fees from Fresenius Kabi.